Session Information
Date: Tuesday, November 14, 2023
Title: (2039–2060) Pediatric Rheumatology – Clinical Poster III: Potpourri
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with Neonatal-Onset Multisystem Inflammatory Disease (NOMID) often require long-term high-dose treatment with anakinra at 5-8 mg/kg daily subcutaneous injections to control CNS inflammation that includes aseptic meningitis, hydrocephalus, and sensorineural hearing loss. We recently described anakinra-associated amyloidomas that present with “fixed subcutaneous lumps” at the site of anakinra injections in patients with NOMID after 13 and 16 years of treatment 1 and screened our cohort for development of iatrogenic (Interleukin-1 receptor antagonist protein)-type amyloid (anakinra) (AIL1RAP).
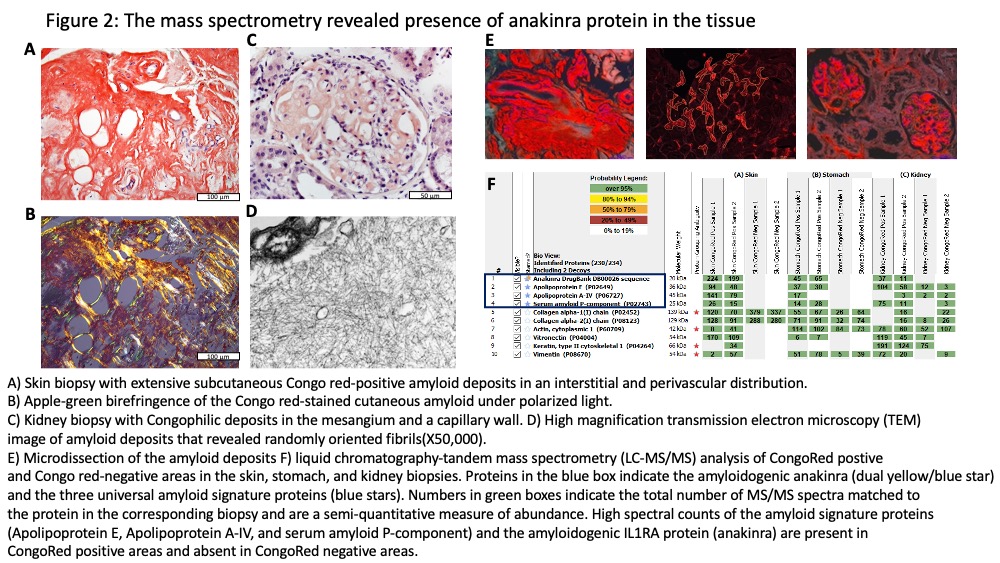
Methods: Telephone screening of 41 NOMID pts enrolled in NCT02974595 identified 10 pts with fixed subcutaneous lumps at the anakinra injection site. Of those six patients underwent skin biopsies, clinical assessment of treatment response, and serum IL-1 receptor antagonist measurements by ELISA, which measures endogenous and recombinant IL-1 receptor antagonist (anakinra). Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed to chemically characterize microdissected amyloid deposits in skin biopsies.
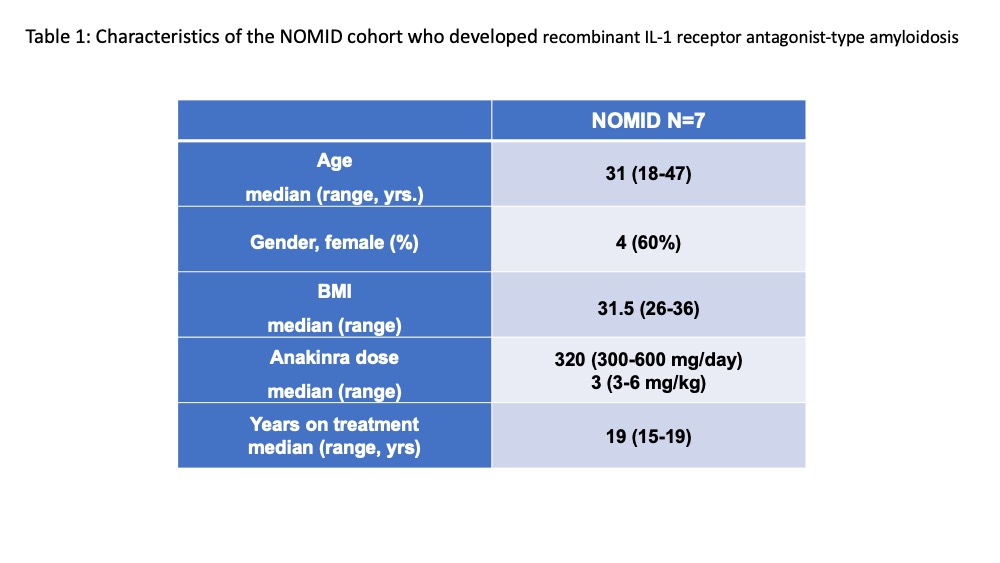
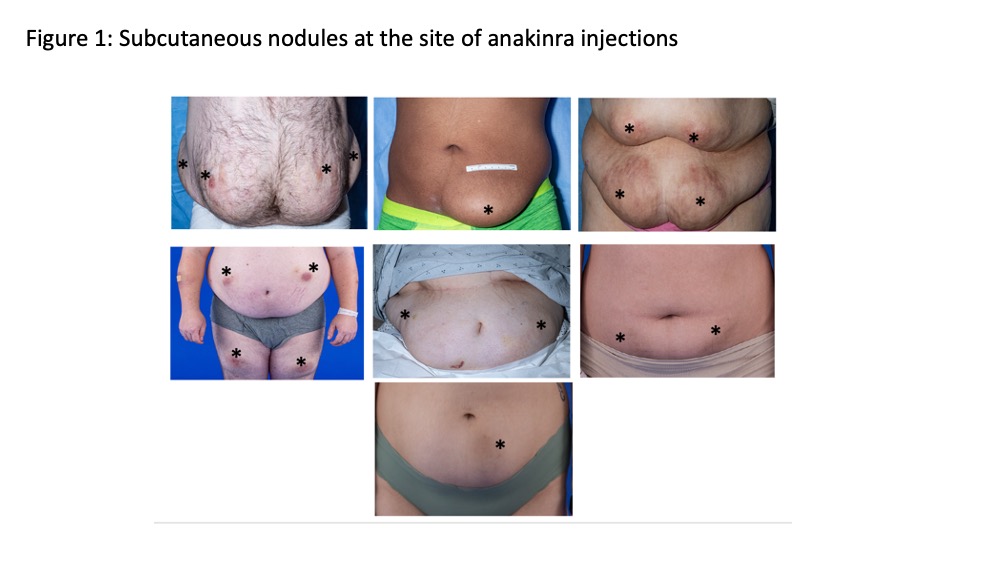
Results: All seven patients biopsied had amyloid deposits at the deep dermis that by mass spectrometry was identified as recombinant of anakinra-type amyloid (AIL1RAP)2 (Figure 1). All patients had been on a median 19 yrs. of treatment and received a median dose of 320 mg (3 syringes) daily. Table 1. All were in clinical remission with normal CRPs. One patient with nephrotic range proteinuria and early satiety had kidney and gastric biopsies that showed AIL1RAP deposits in glomeruli, the tubular basement membrane, and in-between glands in the gastric mucosa. The six other patients had no clinical evidence of disseminated amyloidosis. Serum IL-1ra levels (n=6) ranged from 7500 to 18,000 ng/mL, compared levels between 100-400 pg/mL in healthy controls (n=11). Patients with the focal form of the AIL1RAP amyloidosis were switched to the combination of canakinumab injections every 4 wks and anakinra injections between 50-200 mg daily, that were required to achieve control of headache and CNS inflammation. The patient with systemic Amyloidosis only received canakinumab but developed headaches. The CSF evaluation showed a rise in the IL-6 (from 2 pg/mL on anakinra alone before the switch) to 403 pg/mL (nl< 7.5 pg/mL) on canakinumab monotherapy (300mg/month).
Conclusion: AIL1RAP skin amyloidosis developed in 7 NOMID patients (20 % of screened patients) on high-dose long-term, anakinra treatment; one patient developed systemic AIL1RAP amyloidosis. Combination of canakinumab with lower anakinra doses, controlled CNS inflammation however “safe” serum levels that prevent the development and dissemination iatrogenic AIL1RAP amyloidosis have not been established. Screening all patients, early diagnosis of AIL1RAP-type amyloid and treatment adjustments are critical in assuring adequate treatment and in reducing the risk of developing systemic AIL1RAP amyloidosis.
To cite this abstract in AMA style:
Alehashemi S, Metpally A, Dasari S, Uss K, Hathaway L, Kuhns D, Fink D, Lee C, Castelo-Soccio L, Cowen E, Nasr S, McPhail E, Goldbach-Mansky R. Management of Iatrogenic, Recombinant Interleukin-1 Receptor Antagonist-type Amyloidosis on NOMID in Patients on Anakinra [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/management-of-iatrogenic-recombinant-interleukin-1-receptor-antagonist-type-amyloidosis-on-nomid-in-patients-on-anakinra/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/management-of-iatrogenic-recombinant-interleukin-1-receptor-antagonist-type-amyloidosis-on-nomid-in-patients-on-anakinra/