Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with SLE have a high risk of infections, which remains a common cause of mortality in this population.1 This infection risk can result from the pathophysiology of SLE itself, with corticosteroids and immunosuppressive therapy contributing to further infection risks.1 Belimumab is an approved treatment for active SLE and LN, in addition to standard therapy.2 To evaluate the incidence of infections onbelimumab treatment, we summarized 52-week infection data from placebo-controlled belimumab SLE studies.
Methods: Infection data were sourced from the Phase 4 Belimumab Assessment of Safety in SLE (BASE) study (GSK Study BEL115467)3 and GSK Study BEL116559, a pooled post hoc analysis of 52-week safety data from six studies (BEL110751,4 BEL110752,5 BEL112341,6 BEL113750,7 BEL115471,8 and LBSL029). In BASE, 4003 adults with active, autoantibody-positive SLE (no criteria based on disease activity level) received belimumab (10 mg/kg/month intravenously [IV]) or placebo, plus standard therapy (ST), for 48 weeks. Only data for patients receiving marketed doses of belimumab (10 mg/kg/month IV or 200 mg/week subcutaneously) or placebo, plus ST are reported from BEL116559 integrated randomized controlled trials (RCTs) included in this analysis, with criteria to exclude patients with low disease activity at baseline. The number of serious infections and infections of special interest (opportunistic infections, active tuberculosis, herpes zoster, sepsis) through 52 weeks were summarized for belimumab and placebo treatment groups.
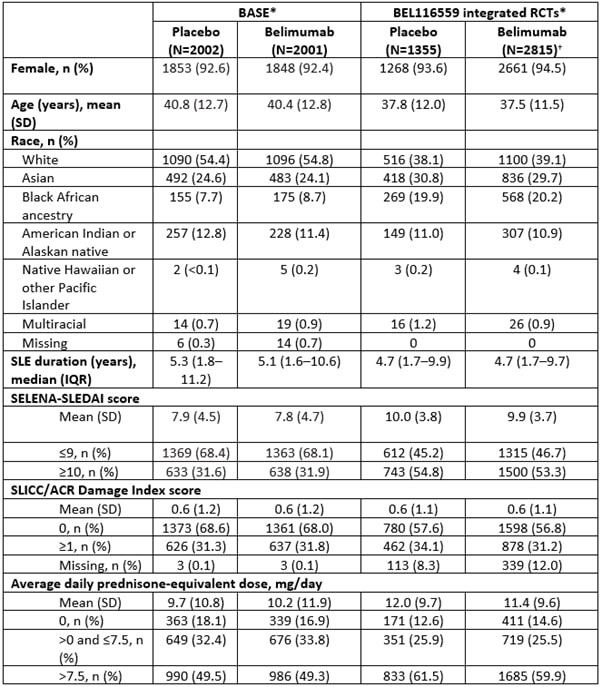
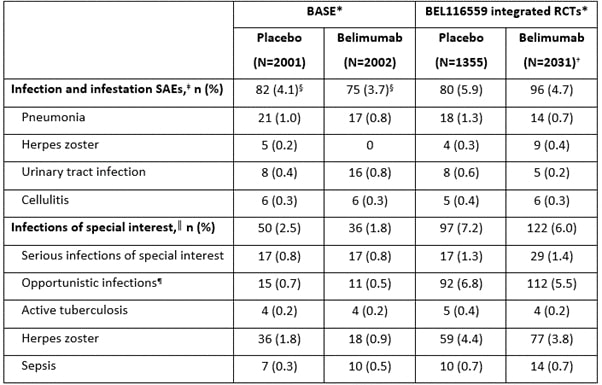
Results: The age and sex profile of patients in the BASE and integrated BEL116559 studies were similar; however, in BASE more patients were white, the median disease duration was longer, and disease activity and average doses of corticosteroids were lower than in BEL116559 (Table 1). Over 52 weeks, serious adverse events (SAEs) of infection and infestation occurred slightly more frequently with placebo than belimumab in both BASE and BEL116559 (Table 2). Infections of special interest were of a similar or higher incidence (such as with opportunistic infections and herpes zoster) in BEL116559 versus BASE and occurred more frequently with placebo than belimumab in both datasets (Table 2).
Conclusion: Over 52 weeks, serious infections and infections of special interest occurred at a similar or higher incidence with placebo versus belimumab in the Phase 4 BASE safety study and the BEL116559 pooled analysis of safety RCT data. These data further support the favorable safety profile of belimumab for SLE treatment.
Funding: This analysis of the GSK Studies 115467 and 116559 was funded by GSK.
References:
1 Yuan Q et al. Semin Arthritis Rheum 2020;50(5):1022–39
2 GSK. Benlysta US prescribing information. 2023
3 Sheikh SZ et al. Lancet Rheum 2020;3:e122–30
4 Furie R et al. Arthritis Rheumatol 2011;63(12):3918–30
5 Navarra SV et al. Lancet 2011;377(9767):721–31
6 Stohl W et al. Arthritis Rheumatol 2017;69(5):1016–27
7 Zhang F et al. Ann Rheum Dis 2018;77(3):355–63
8 Ginzler E et al. Arthritis Rheumatol 2021; doi: 10.1002/art.41900
9 Wallace DJ et al. Arthritis Rheum 2009;61(9):1168–78
*Baseline patient characteristics are presented for the intention-to-treat population; †patient characteristics for integrated randomized-controlled trials include patients on all doses of belimumab; this cohort is not limited to patients receiving marketed doses (10 mg/kg intravenous or 200 mg subcutaneous) of belimumab.
IQR, interquartile range; SD, standard deviation; SELENA, Safety of Estrogens in Lupus Erythematosus National Assessment.
*BASE and BEL116559 safety data are shown for the on-treatment population, patient numbers vary between baseline data and Week 52 follow-up data, since patients could withdraw from study agent during Year 1 and continue on study, or could withdraw from Year 1 study visits and reconsent to a Week 52 follow-up; †this cohort is limited to patients receiving marketed doses (10 mg/kg intravenous or 200 mg subcutaneous) of belimumab; ‡data for the 4 most common individual SAEs for the BASE and BEL116559 datasets are shown, calculated as the total incidence across both treatment arms; §only treatment-emergent SAEs for the on-treatment period are summarized from patients who received ≥1 dose of belimumab or placebo; ║all infections of special interest are limited to opportunistic infections, active tuberculosis, herpes zoster, and sepsis; ¶per sponsor adjudication.
To cite this abstract in AMA style:
Sheikh S, Withrop K, Yazdany J, Navarra S, Atsuma T, Curtis P, Olaiz J, Henning C, Levy R, Stohl W, Wallace D. Cumulative Infections by Week 52 Among Patients with SLE: A Summary of Data from Placebo-Controlled Belimumab Studies [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/cumulative-infections-by-week-52-among-patients-with-sle-a-summary-of-data-from-placebo-controlled-belimumab-studies/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cumulative-infections-by-week-52-among-patients-with-sle-a-summary-of-data-from-placebo-controlled-belimumab-studies/