Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Pooled safety data have been reported for secukinumab administration in patients with psoriasis (PsO), psoriatic arthritis (PsA) and axial spondyloarthritis (axSpA).1,2 The aim of this study is to describe the safety profile of secukinumab after extensive patient exposure in clinical trials. We report safety analysis from a larger pool of patients and more studies than previously published.3
Methods: The pooled safety analysis included 48 Phase II/III/IV clinical trials with patients who had received subcutaneous (s.c.) secukinumab 150 mg and / or 300 mg for at least 16 weeks in PsO (31 trials), PsA (9 trials), and axSpA (8 trials), with a cut-off date of June 2022. AxSpA includes data from patients with either radiographic axSpA or non-radiographic axSpA. Adverse events (AEs) were reported as exposure-adjusted incidence rates (EAIRs) per 100 patient-years. Analysis included all patients who received ≥1 dose of secukinumab.
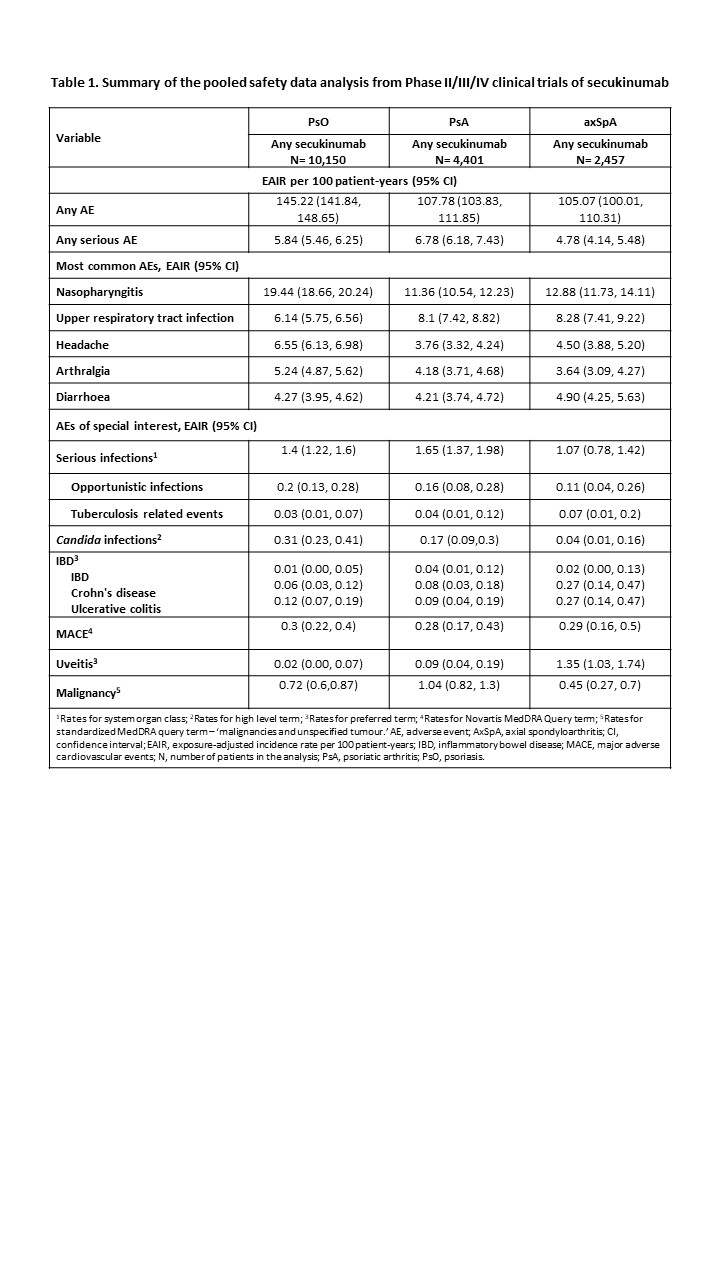
Results: A total of 17,008 patients with PsO (10,150), PsA (4,401) and axSpA (2,457) were included in this analysis. The most frequent AEs reported across all indications were nasopharyngitis (EAIR [95% CI]; PsO, 19.44 [18.66, 20.24]; PsA, 11.36 [10.54, 12.23]; axSpA, 12.88 [11.73, 14.11]) and upper respiratory tract infection (EAIR [95% CI]; PsO, 6.14 [5.75, 6.56]; PsA, 8.1 [7.42, 8.82]; axSpA, 8.28 [7.41,9.22]). EAIRs per 100 patient-years for inflammatory bowel disease (IBD), malignancies and major adverse cardiovascular events (MACE) remained low across all indications. The EAIRs per 100 patient-years for adverse events (AEs) of special interest are reported in Table 1.
Conclusion: This pooled safety data analysis of 48 Phase II/III/IV clinical trials demonstrates that secukinumab is well tolerated in patients with PsO, PsA and axSpA, and shows no new signals with longer-term follow-up beyond those already identified in shorter studies.1,2
References
1. Deodhar et al. Arthritis Research & Therapy (2019) 21:111.
2. Deodhar et al. EULAR (2020). Eposter no. FRI0272.
3. Gottlieb et al. Acta Derm Venereol (2022) 102: adv00698.
To cite this abstract in AMA style:
Deodhar A, McInnes I, Baraliakos X, Gottlieb A, Kiltz U, Schreiber S, Gopal Sahoo B, Bao W, Richards H, Pricop L, Gaillez C, Dong V, Mease P. Secukinumab Demonstrates a Consistent Safety Profile in Patients with Psoriasis, Psoriatic Arthritis and Axial Spondyloarthritis: Updated Pooled Safety Analysis from Clinical Trials [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/secukinumab-demonstrates-a-consistent-safety-profile-in-patients-with-psoriasis-psoriatic-arthritis-and-axial-spondyloarthritis-updated-pooled-safety-analysis-from-clinical-trials/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-demonstrates-a-consistent-safety-profile-in-patients-with-psoriasis-psoriatic-arthritis-and-axial-spondyloarthritis-updated-pooled-safety-analysis-from-clinical-trials/