Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor-alpha (TNF-α) inhibitors, a widely used class of biological immunomodulating agents targeting TNF-α, have revolutionized the treatment of various autoimmune and inflammatory conditions. However, emerging evidence suggests a potential association between TNF-α inhibitors and demyelination, a critical factor in the development and progression of neurological disorders. This abstract analyzes data from the FDA Adverse Event Reporting System (FAERS) and helps understand the potential risks underlying this association.
Methods: We performed a retrospective pharmacovigilance study that analyzed the FAERS database from 2009-2023 to investigate the association of demyelinating adverse events (AE), namely Guillain-Barre syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), transverse myelitis (TM), multiple sclerosis (MS), optic neuritis (ON), and progressive multifocal leukoencephalopathy (PML) in patients treated with FDA-approved TNF-α inhibitors (infliximab, adalimumab, etanercept, golimumab, and certolizumab). Disproportionality analysis was performed using reporting odds ratio (ROR).
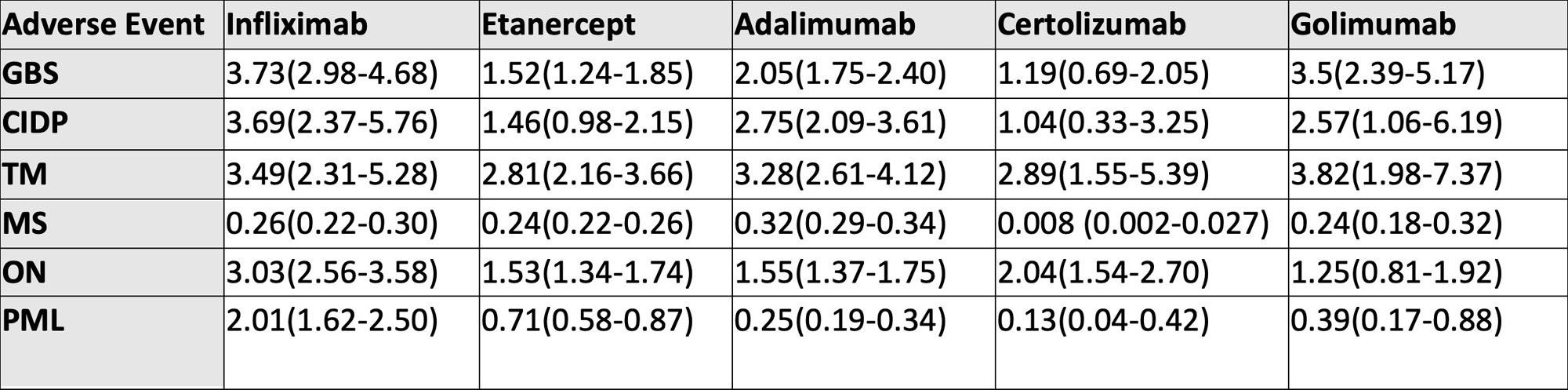
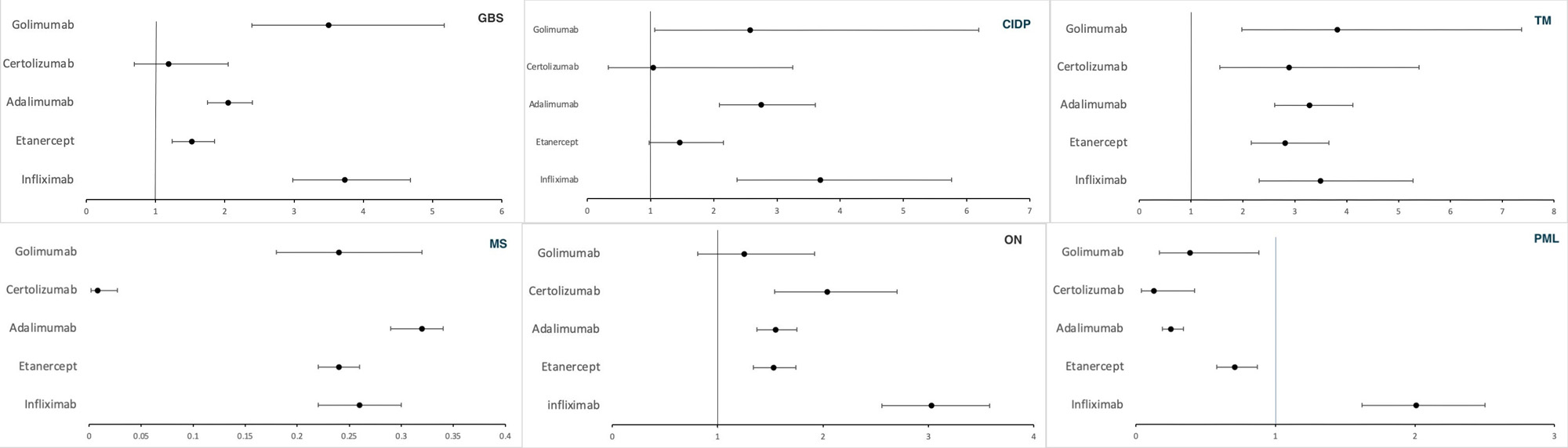
Results: A total of 1,372,262 AEs were reported with TNF-α inhibitors, of which 170,109 were neurological AEs. Most AEs were among patients in the 18-64 age group (38-64%). The death rate was 4-7 % for all demyelinating categories except PML, in which the death rate was 18-22% with infliximab, etanercept, and adalimumab. GBS, CIDP, ON, and PML were disproportionately associated with the use of infliximab with a ROR (95% CI) of 3.73 (2.98-4.68), 3.69 (2.37-5.76), 3.03 (2.56-3.58) and 2.01 (1.62-2.50) respectively. Golimumab had the highest reported association with TM, with a ROR of 3.82 (1.98-7.37). We observed a negative association of all TNF-α inhibitors with MS (ROR< 1).
Conclusion: Our study found that using certain biologic agents is associated with an increased risk of specific demyelinating disorders. While a temporal relationship between anti-TNF-α treatment and demyelinating events is suggested, the overall number of published cases is small compared to the total number of treated patients. In most cases, demyelination either progressed slowly or resolved after discontinuing anti-TNF-α therapy, indicating a potential protective effect or short-lasting harmful impact in patients already suffering from latent MS. Genetic predisposition and overlapping syndromes in autoimmune diseases also cast doubt on a direct relationship. We suggest a thorough neurological assessment of candidates considered for TNF-α blockers before initiation and periodically during treatment and avoiding the use in patients with a history or family history of demyelinating diseases. Limitations of the study include reporting bias, confounding of the observed associations, and the observational nature of the study that prevents establishing causal relationships. Further controlled studies, such as randomized clinical trials or prospective cohort studies, are needed to confirm our findings and establish causality.
To cite this abstract in AMA style:
Sondhi M, Vyas R, Thakre A, Hayat S. Exploring the Risk of Demyelination Associated with TNF Alpha Inhibitors: Analysis of the FDA Adverse Event Reporting System (FAERS) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/exploring-the-risk-of-demyelination-associated-with-tnf-alpha-inhibitors-analysis-of-the-fda-adverse-event-reporting-system-faers/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-the-risk-of-demyelination-associated-with-tnf-alpha-inhibitors-analysis-of-the-fda-adverse-event-reporting-system-faers/