Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Autoimmune retinopathy (AIR) is a disease process in which circulating autoantibodies (AAbs) against retina-specific antigens cause local inflammation and can lead to blindness. Hydroxychloroquine (HCQ), likewise, can cause retinal toxicity, typically in a dose-dependent manner, for which routine screening is recommended in systemic lupus erythematosus (SLE) patients. AIR has been reported in patients with SLE but the frequency of antiretinal AAbs in SLE patients is not well described. Furthermore, the role of antiretinal AAbs in HCQ related eye toxicity has not been described. The objective of this study is to determine whether patients diagnosed with HCQ retinal toxicity are more likely to have circulating antiretinal AAbs compared to controls.
Methods: This study was conducted at two academic institutions within the United States. Patients in this study were enrolled in a longitudinal cohort study of cardiovascular risk factors. We performed antiretinal AAbs testing on 269 SLE plasma samples collected at baseline. We retrospectively reviewed charts of these patients for the presence of HCQ retinal toxicity, as well as the baseline presence of risk factors for HCQ toxicity. Our primary goal was to determine frequency of specific antiretinal AAbs in SLE patients with HCQ retinal toxicity compared to SLE patients without retinal toxicity.
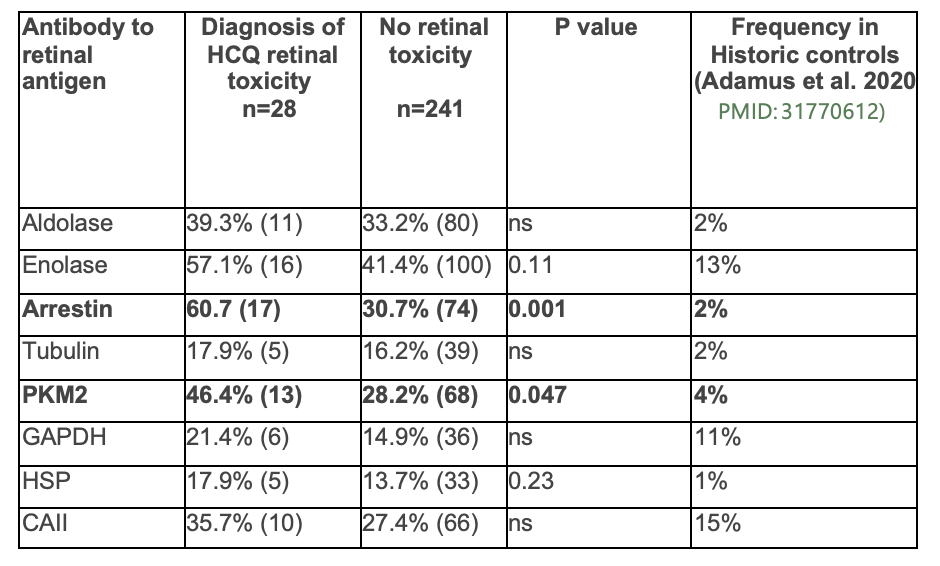
Results: Patients with a diagnosis of HCQ retinal toxicity had a higher likelihood of testing positive for anti-arrestin AAbs (60.7% of patients vs. 30.7% of patients, p=0.001) and PKM2 antibodies (46.4% of patients vs. 28.2% of patients, p=0.047) compared to patients without a diagnosis of retinal toxicity (see table 1). Patients with a history of HCQ eye toxicity also had a trend towards a higher number of AAbs, with a mean of 2.96 ± 2.40 vs. 2.05 ± 1.74 in the group with no history of toxicity. In multivariate analysis accounting for risk factors associated for HCQ eye toxicity, including renal disease, BMI, average HCQ dose per year of disease duration, and disease severity using the Lupus Severity Index (LSI), we found that the presence of arrestin AAbs was associated with OR 3.2 for developing HCQ eye toxicity.
Conclusion: Antiretinal AAbs, especially arrestin and PKM2, were more common in SLE patients with HCQ retinal toxicity compared to patients without HCQ retinal toxicity. The pathogenesis and clinical significance of these autoantibodies is not clear. However, even when controlling for other risk factors associated with HCQ eye toxicity, anti-arrestin AAbs were associated with increased odds for the development of eye toxicity, suggesting a potential role for antiretinal AAbs as a biomarker of HCQ eye toxicity risk. Further prospective studies are needed to evaluate the risk of developing HCQ retinal toxicity in patients with known circulating antiretinal AAbs.
To cite this abstract in AMA style:
Good S, Adamus G, Gorin M, Jacquez J, Grossman J, Skaggs B, Hasan A, McMahon M. Antiretinal Autoantibodies in Hydroxychloroquine Eye Toxicity [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/antiretinal-autoantibodies-in-hydroxychloroquine-eye-toxicity/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/antiretinal-autoantibodies-in-hydroxychloroquine-eye-toxicity/