Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: PsA
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: To investigate the long-term efficacy, safety, and tolerability of intravenous (IV) secukinumab (SEC) in patients with active psoriatic arthritis (PsA) through 52 weeks.
Methods: INVIGORATE-2 (NCT04209205) is a randomized, double blind, placebo (PBO)-controlled, parallel group, phase 3 study. All patients met the CASPAR criteria for active PsA, had symptoms for ≥6 months, and had a Tender Joint Count of 78 joints (TJC78) ≥3 and a Swollen Joint Count of 76 Joints (SJC76) ≥3 at baseline. Patients were randomized 1:1 to receive IV SEC (6 mg/kg at baseline followed by 3 mg/kg q4w) or PBO. At Week 16, patients randomized to SEC continued receiving SEC and patients randomized to PBO were switched to IV SEC (3 mg/kg q4w) through Week 52. The primary efficacy endpoint was American College of Rheumatology (ACR) 50 response rate at Week 16 versus PBO. Secondary efficacy outcomes included achievement of ACR20, minimal disease activity, PASI90, dactylitis resolution, and enthesitis resolution. All efficacy outcomes were assessed through Week 52, and safety was evaluated through Week 60. Data through week 16 are reported using nonresponder imputation; data from Week 20 through Week 52 are reported as observed.
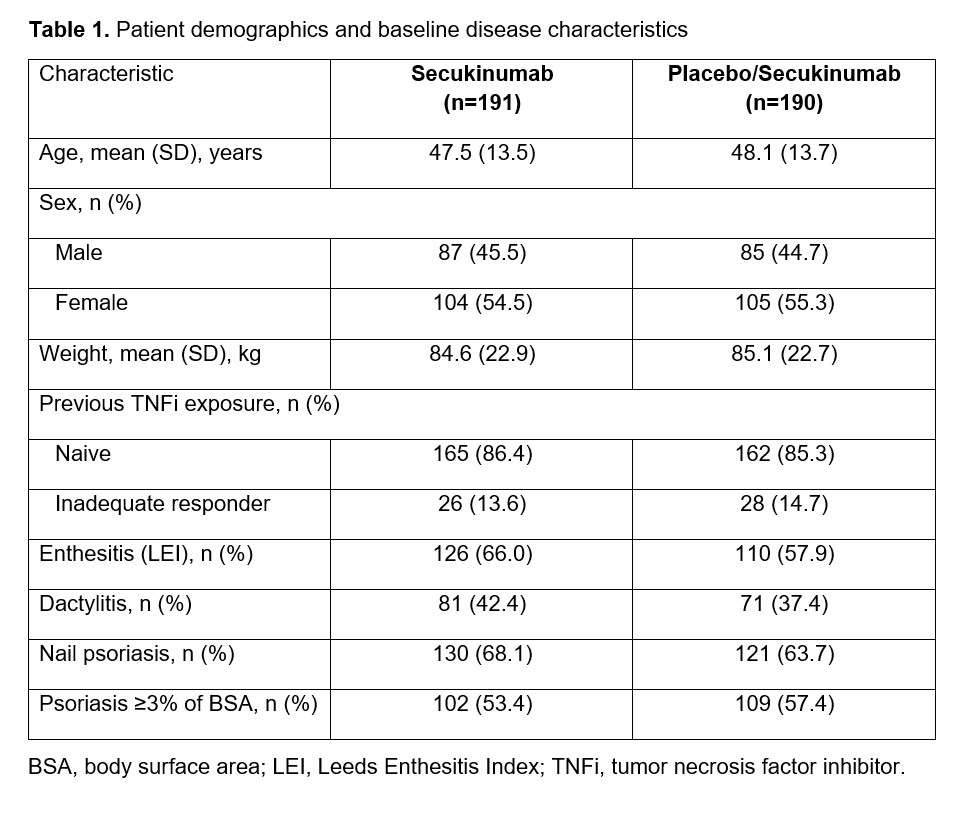
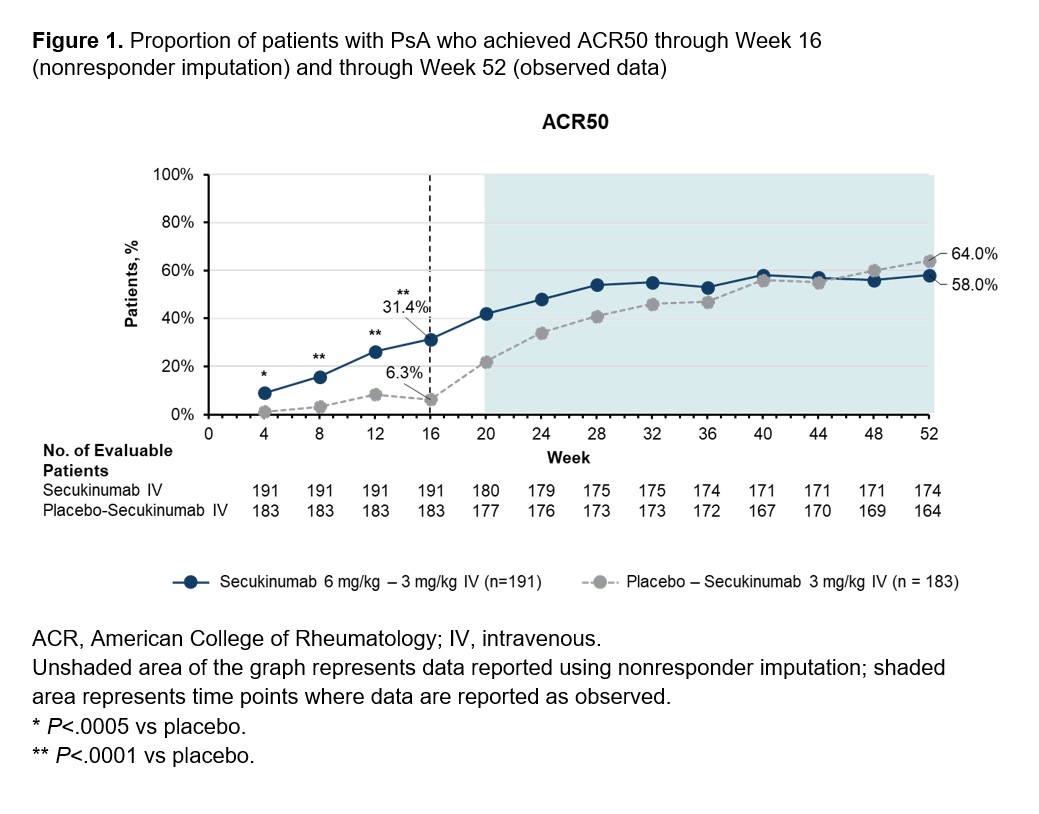
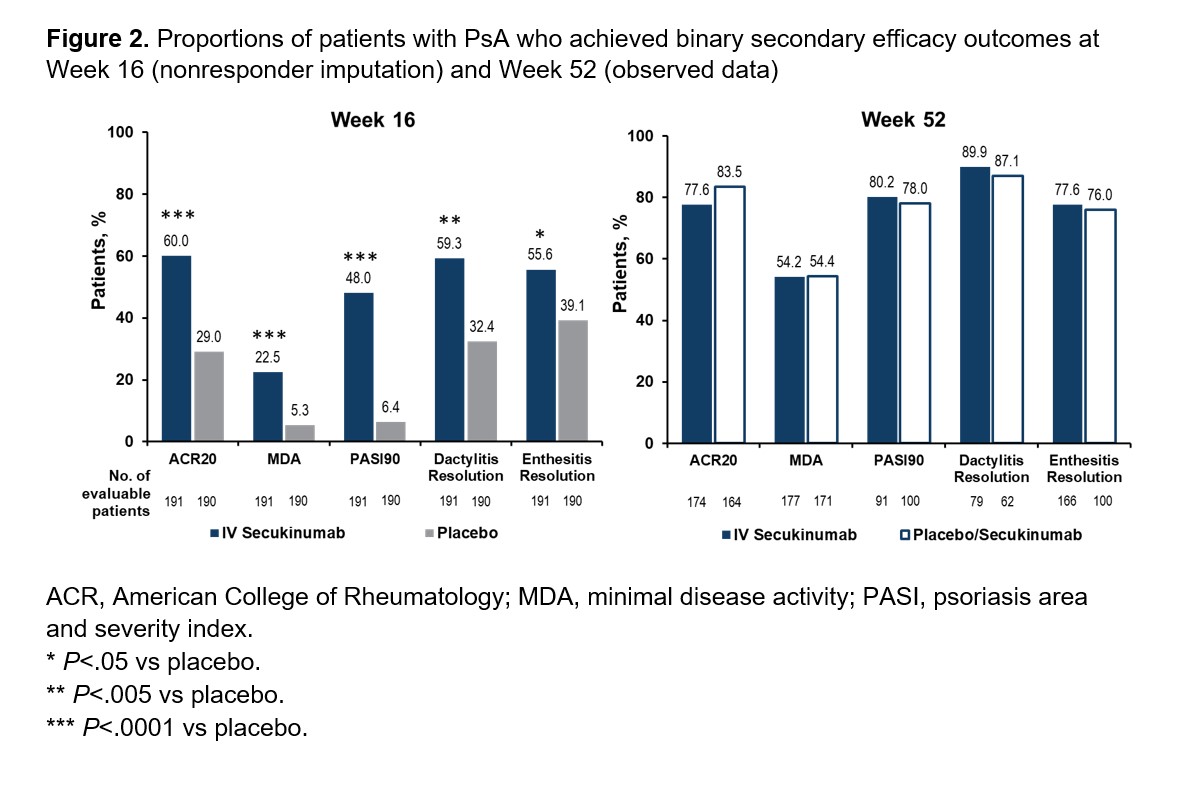
Results: Demographics and baseline characteristics were balanced across treatment groups (Table 1). Among patients randomized to IV SEC (n=191) and IV PBO/SEC (n=190), similar proportions of patients completed the entire study period (90.6% and 87.9%, respectively). A higher proportion of patients receiving SEC than PBO achieved ACR50 at Week 16 using nonresponder imputation (60/191 [31.4%] vs 12/190 [6.3%]; P< .0001; Figure 1). An increase in ACR50 response was observed by Week 20 among patients initially randomized to PBO who were switched to SEC at Week 16. Both groups had similar ACR50 response rates by Week 52 based on observed data (SEC, 58.0%; PBO/SEC, 64.0%). Patients receiving SEC had greater improvements in secondary efficacy outcomes compared with PBO at Week 16, and both SEC and PBO/SEC groups had comparable efficacy outcomes by Week 52 (Figure 2). Through Week 16, the incidences of adverse events (AEs; 37.7% vs 38.4%), serious AEs (1.6% vs 2.1%), and AEs leading to treatment discontinuation (0.5% vs 1.6%) were similar for patients receiving SEC or PBO. Among patients receiving any SEC through the entire study period, 63.4%, 5.9%, and 1.9% reported any AE, serious AEs, and AEs leading to treatment discontinuation, respectively. One death was reported in the PBO group prior to Week 16. No new safety signals were observed with IV SEC.
Conclusion: IV SEC (6 mg/kg at baseline followed by 3 mg/kg q4w) was safe and effective for the long-term treatment of active PsA. Treatment responses were maintained up to Week 52 for patients randomized to IV SEC. For patients originally randomized to PBO who switched to receive IV SEC at Week 16, an increase in efficacy responses comparable to those in patients randomized to IV SEC was observed up to Week 52. Safety was consistent with the known safety profile of subcutaneous SEC, and no new safety signals were observed.
To cite this abstract in AMA style:
Kivitz A, Sedova L, Churchill M, Kotha R, Singhal A, Torres A, Valenzuela G, Whelan S, Dumortier T, Zhu X, Martin R, Pricop L. Efficacy and Safety of Intravenous Secukinumab for the Treatment of Active Psoriatic Arthritis: 16- and 52-Week Results from a Randomized, Double-Blind, Phase 3 Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/efficacy-and-safety-of-intravenous-secukinumab-for-the-treatment-of-active-psoriatic-arthritis-16-and-52-week-results-from-a-randomized-double-blind-phase-3-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-intravenous-secukinumab-for-the-treatment-of-active-psoriatic-arthritis-16-and-52-week-results-from-a-randomized-double-blind-phase-3-study/