Session Information
Date: Sunday, November 12, 2023
Title: (0691–0721) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The burden of continuing glucocorticoids (GCs) in patients with polymyalgia rheumatica (PMR) who have an inadequate response (IR)to GCs has not been evaluated.
Methods: An inception cohort of adult patients with a new diagnosis of PMR was created using fee-for-service Medicare claims data from 2016 to 2020. Patients with PMR were included if they were aged≥50 and had 1)≥1 inpatient or ≥2 outpatient claims with ICD-10-CM M35.3 ≥30 days apart, 2) a prescription for GC 7.5–25 mg/day <30 days from the 1stinpatient code or between 1stoutpatient code to 30 days after the 2ndcodewith cumulative use of≥200 mg over ≥120 days, and 3) continuous enrollment ≥1 year prior (baseline) to index date, with no evidence for PMR. The index date was the later of the 2nd outpatient PMR diagnosis, or the inpatient PMR diagnosis; and the date that GC dose/use criteria was met; and had to fall between 10/1/2016 to 12/31/2019. Exclusion criteria included baseline diagnosis of seropositive rheumatoid arthritis, other systemic rheumatic disease (including giant cell arteritis),organ transplant, multiple sclerosis, or active treatment of malignancy or who had any prescription for conventional immunomodulatory therapy or an interleukin-6 receptor inhibition at baseline. Patients were classified as GC inadequate responders (GC–IRs), defined as use of ≥7.5 mg/day at 6 months (i.e., presumed flare), continued use of GCs >12 months, or relapse (re-initiation of GC after >60-day gap discontinuation) or non-GC–IR.
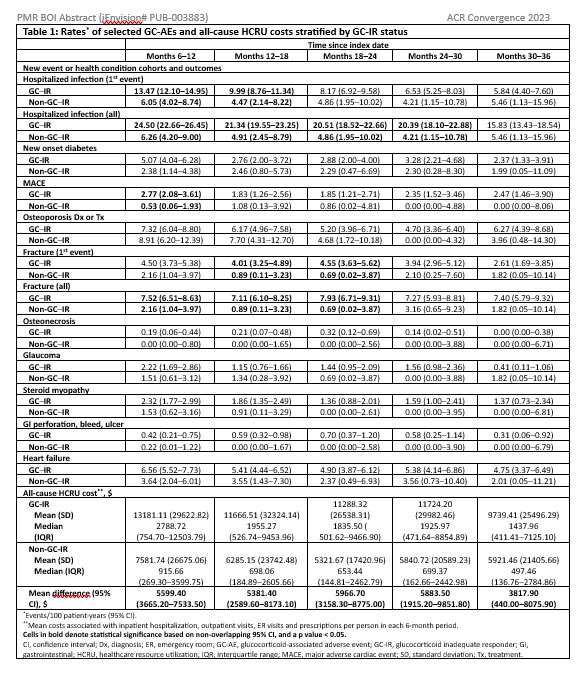
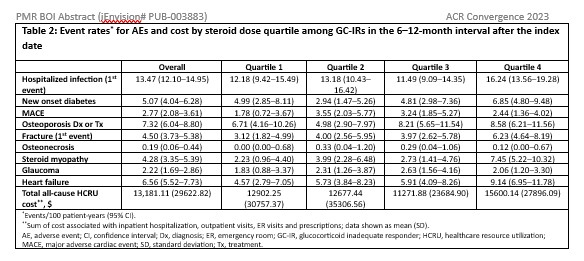
All-cause healthcare resource utilization cost and event rate of pre-specified, GC-associated adverse events (GC-AEs) that were defined by ICD-10-CM diagnosis codes, medication use, and/or Healthcare Common Procedure Coding System medical procedures beginning 6 months after the index date and updated in 6-month intervals were reported. Adverse events (AEs) were identified using claims-based algorithms that have been previously used or reported in the literature. For GC-AEs, with the exception of hospitalized infections and fractures, only the 1stevent was counted. Event rates and 95% confidence intervals were estimated using Exact Poisson methods. A subgroup analysis of the GC–IR cohort was performed, estimating outcomes by cumulative steroid dose quartiles received; dose quartiles were updated at 6 months.
Results: A total of 6,054 patients with PMR met eligibility criteria:mean age 77.0 years, 65.4% women, 96.1% White, and 3.0% Hispanic. The rate of GC-AEs and medical cost were consistently higher in the GC–IR versus non-GC–IR through 18 months, except for osteoporosis (Table 1). In the GC–IR cohort, cost and most GC-AEs suggested a decrease over time, likely due to GC discontinuation or lower doses of GC, and/or for AE-related censoring (i.e., depletion of at-risk patients). Further, most outcomes showed a dose-response relationship between cumulative GC dose and outcomes (Table 2).
Conclusion: There is a substantial clinical and economic burden of continuing GCs in PMR patients with IR. Some GC-AEs and associated costs may be enduring. These data provide insight into the potential benefit of minimizing long-term GCuse and need for more effective GC-sparing therapies.
To cite this abstract in AMA style:
Curtis J, Araujo L, Fiore S, Sattui S, Stone J, Ford K, Xie F. Clinical and Economic Burden of Polymyalgia Rheumatica in Patients with an Inadequate Response to Glucocorticoids in a Real-World Setting [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/clinical-and-economic-burden-of-polymyalgia-rheumatica-in-patients-with-an-inadequate-response-to-glucocorticoids-in-a-real-world-setting/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-and-economic-burden-of-polymyalgia-rheumatica-in-patients-with-an-inadequate-response-to-glucocorticoids-in-a-real-world-setting/