Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rituximab (RTX) is increasingly being employed to treat refractory systemic lupus erythematosus(SLE). Though the drug is effective there is a high risk of adverse events including infections. Most studies have focused on short term(< 1 year) safety, whereas long term follow-up data is sparse. This study was designed to look at the frequency and predictors of infections in patients with SLE receiving RTX who are on long term follow up.
Methods: This was a single-centre retrospective observational study. Data of SLE patients who received at least 1 gram of RTX were abstracted from the case record forms of the department’s biologic registry. Patients were followed up at 3-6 monthly intervals and disease activity, infectious and non-infectious adverse events during or after RTX induction were documented. For those patients who did not have follow up for ≥ 1 year, the last available date of follow-up was considered for this study. Demographic, baseline organ involvement, laboratory data, treatment details were collected. Glucocorticoid exposure was assessed in terms of cumulative steroid (prednisolone) dose, standardized steroid dose (glucocorticoid intake per month of exposure) and number of times the patient received pulse IV methylprednisolone. A ‘serious adverse event’ was defined according to guidelines by International Council on Harmonisation (ICH) 1994. The primary outcome was the occurrence of a serious infection. Secondary outcomes included mortality, time to first serious infection, minor infections and other adverse events.
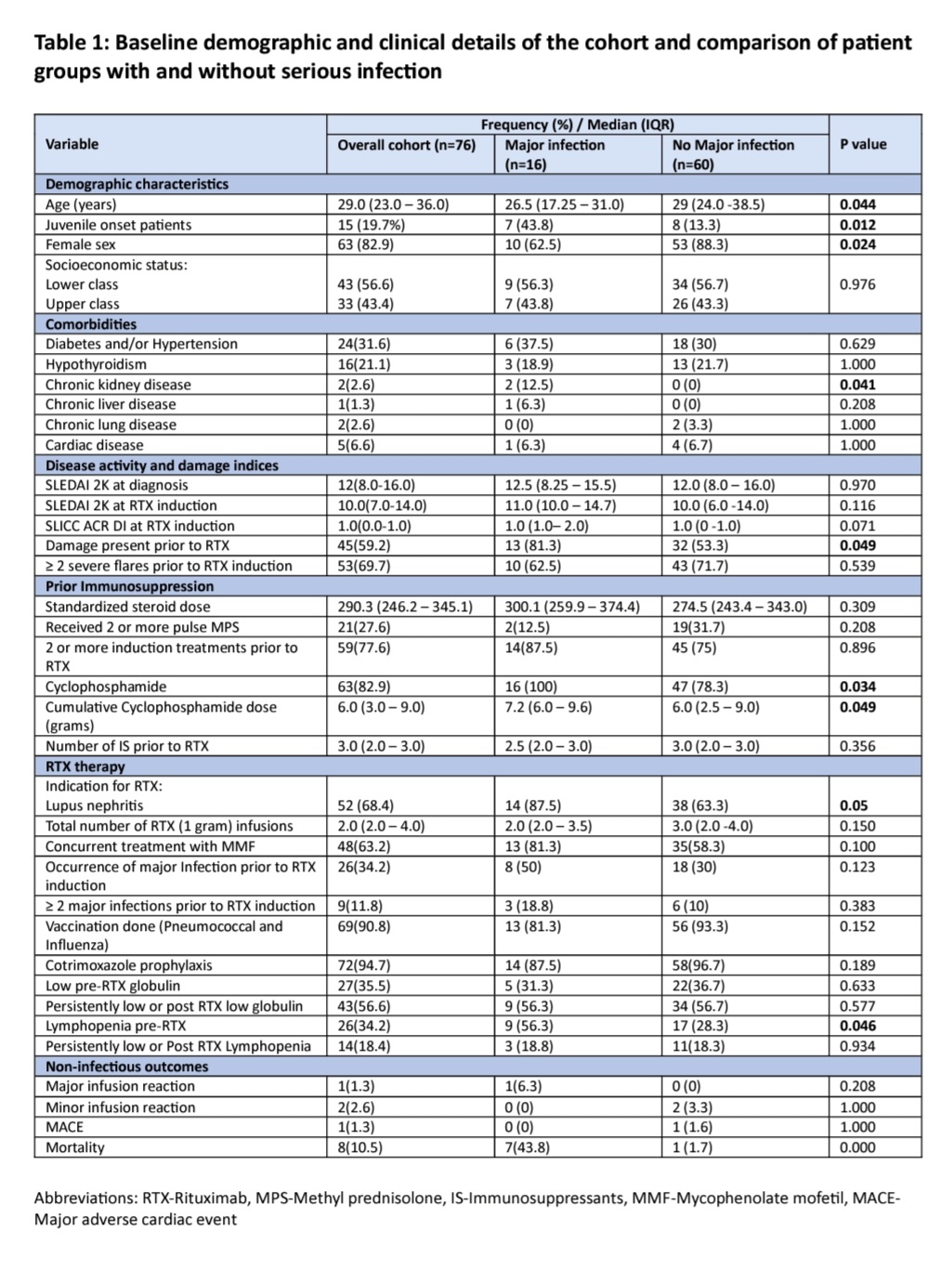
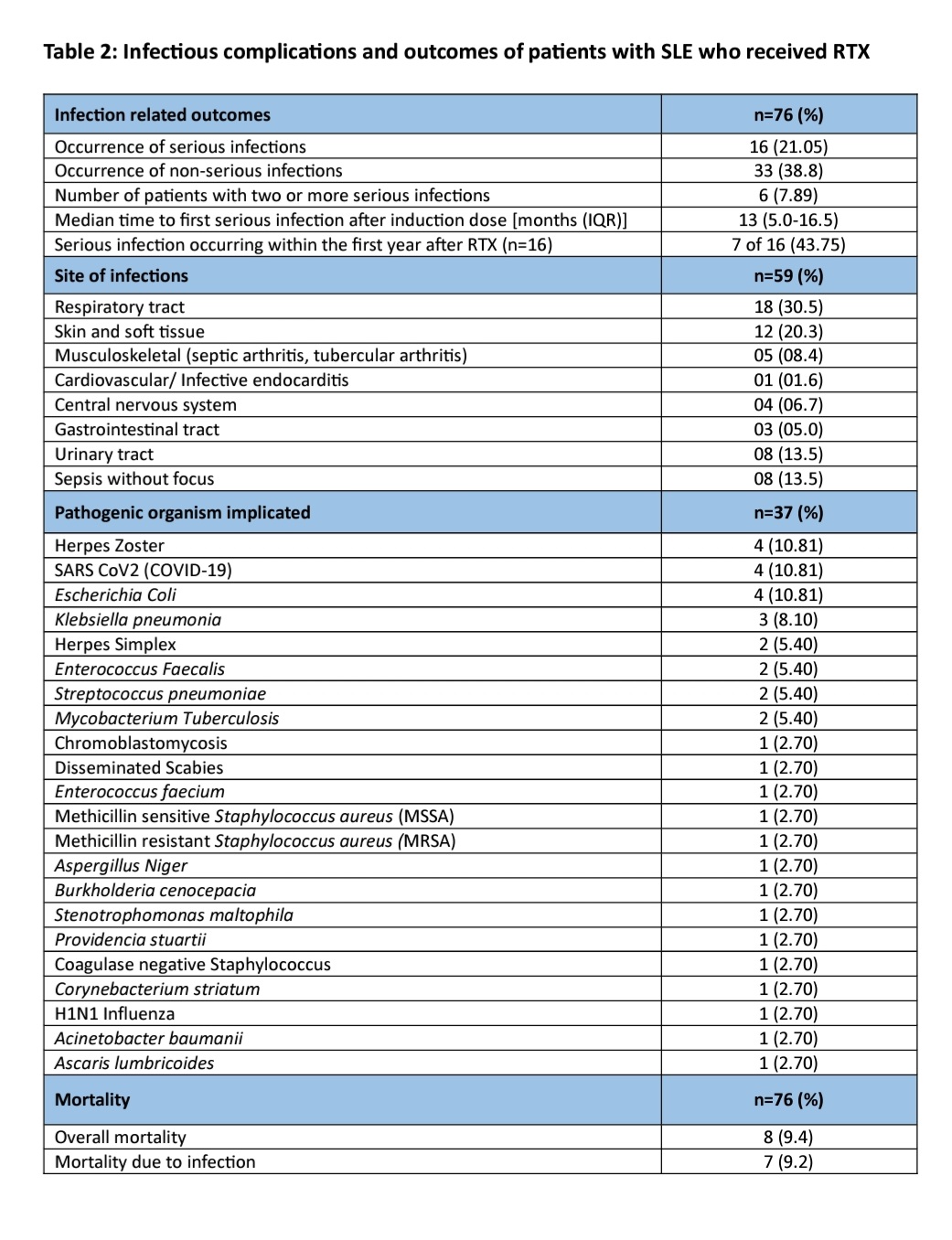
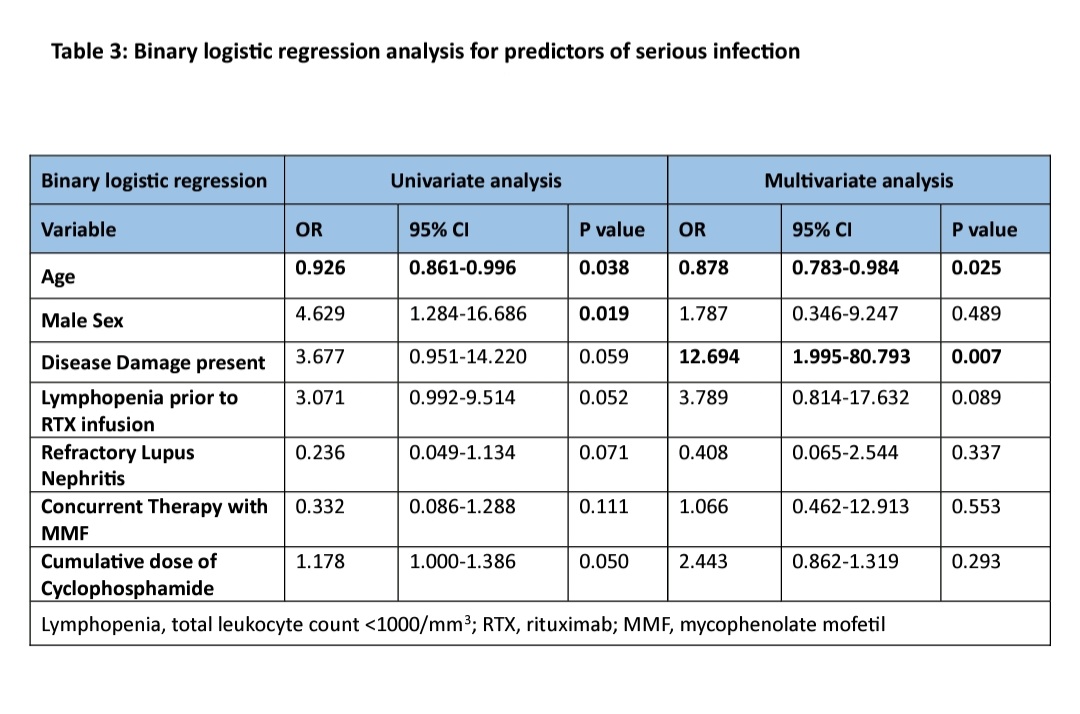
Results: A total of 76 SLE patients (median age 29 years, 83% females) were included. The median follow-up duration was 27.0 (10-49) months amounting to 177.33 person-years of follow-up. Baseline data of the patients is represented in table 1. Forty-five patients (59.2%) had at least one adverse event. Among these, non-infectious events occurred in 4 (5.26%). Infusion reactions occurred 3 (3.9%), one requiring discontinuation of therapy. One patient had worsening of pre-existing pulmonary artery hypertension. There were 43 (56.6%) patients who had infectious events, 16 of 76 (21.05%) were serious infections amounting to 9.02 infections per 100 person-years of follow-up. Majority(53%) of infections occurred after 1 year of RTX infusion. Site of infections and microrganisms implicated are detailed in table 2. There were total 8 deaths resulting in a mortality rate of 4.5 per 100 person-years of follow-up. All deaths except one were due to serious infection. Male sex, younger patients, CKD, previous cyclophosphamide use, higher cumulative dose of cyclophosphamide, refractory lupus nephritis, higher SLICC ACR damage index at baseline was associated with serious infections (table 1). On binary logistic regression younger age(B 95% CI) and presence of disease damage(B 95% CI) emerged as significant predictors of serious infections (table 3).
Conclusion: Serious infections following RTX are common and result in mortality in SLE. More than 50% of them occur after 1 year of RTX administration. Infections arise from common and rare opportunistic organisms. Juvenile lupus and presence of damage at baseline predispose to infections.
groups with and without serious infection
To cite this abstract in AMA style:
Jose A, Gopal A, Gorijavolu M, C Das A, Babu K L J, Nayak A, Kavadichanda C, Mary Thabah M. Long Term Safety and Predictors of Serious Infections Among Patients with Systemic Lupus Erythematosus Treated with Rituximab: Audit from a Single Center Biologic Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-predictors-of-serious-infections-among-patients-with-systemic-lupus-erythematosus-treated-with-rituximab-audit-from-a-single-center-biologic-registry/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-predictors-of-serious-infections-among-patients-with-systemic-lupus-erythematosus-treated-with-rituximab-audit-from-a-single-center-biologic-registry/