Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: We aim to describe the profile of new-onset systemic autoimmune diseases (SAID) developed in individuals following COVID-19 vaccination and to characterize the potential risk factors for their occurrence using a large-scale international survey.
Methods: A retrospective cohort study was conducted among participants who self-reported new-onset SAID in the COVAD-2 study – a global, validated, patient-reported e-survey involving 167 collaborators from 110 countries, to collect data on the long-term safety and tolerability of COVID-19 vaccines in patients with SAID (1). Responses are summarized using descriptive statistics including median (IQR) on the new onset of SAID. Baseline characteristics between those with new-onset SAIDs diagnosed by a rheumatologist and vaccinated healthy controls (HCs) are compared after 1:4 propensity score (PS) matched analysis based on age and gender variables. Predictors of new-onset SAIDs were assessed using binary logistic regression adjusting for age, gender, ethnicity, and country by HDI.
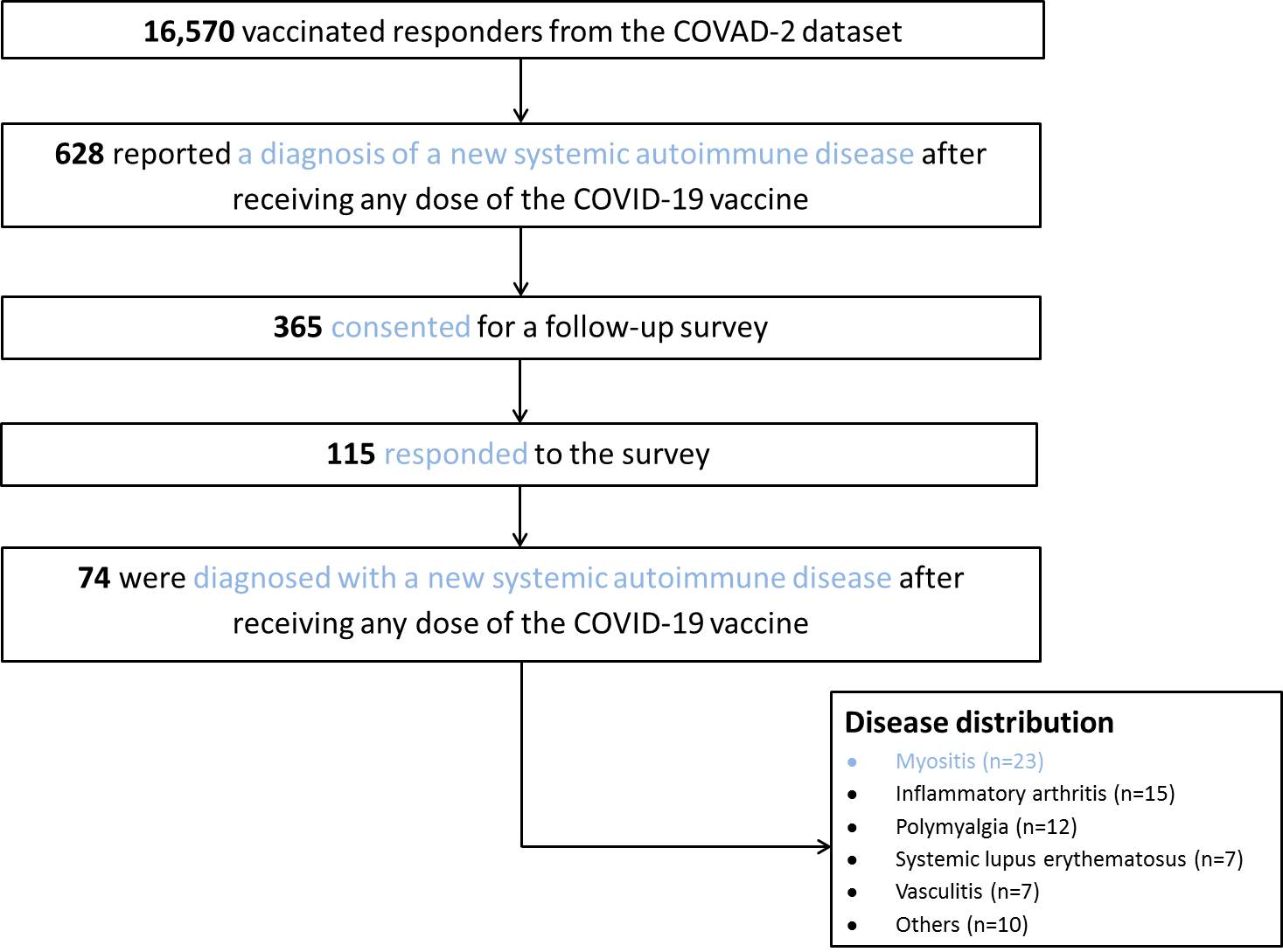
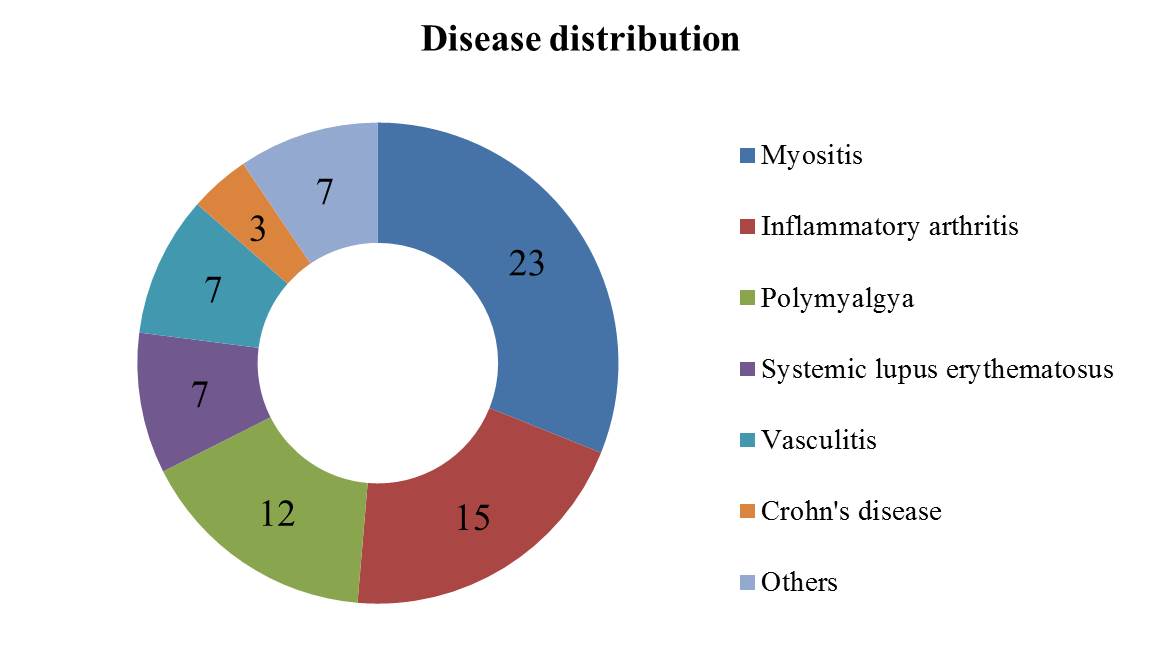
Results: Out of a total of 16,570 vaccinated respondents to the COVAD-2 survey, 628 reported a diagnosis of a new-onset SAID after receiving any dose of a COVID-19 vaccine. Of them 365 consented for a follow-up survey and 115 completed the survey. A total of 74 respondents with confirmed diagnosis of new-onset SAID were included in the final analysis (mean age was 52 years (range 18-82 years; SD: 14) and more than three-quarters were females (n=59, 79.7%)) (fig. 1). The most commonly reported new-onset SAIDs were idiopathic inflammatory myopathies (n=23, 31.1%) followed by inflammatory arthritis (n=15; 20.3%) and polymyalgia rheumatica (n=12, 16.2%) (fig. 2). The most commonly received COVID-19 vaccines leading to new-onset SAID were Pfizer (n=37, 37.4%) followed by Moderna (n=32, 32.3%) and then Oxford/AstraZeneca (n=26, 26.3%). The median (IQR) duration from vaccination to symptom onset was 14 (5-30) days. PS matched analysis between vaccinated HCs and new-onset SAIDs showed higher odds of new-onset SAIDs among Caucasians (OR: 5.3; 95%CI: 2.9-9.7; p< 0.001) and mRNA-1273 (Moderna) vaccine recipients (2.7; 1.3-5.3; 0.004) and lower odds among Asians (0.2; 0.1-0.7; 0.011) and BNT162b2 (Pfizer-BioNTech) vaccine (0.3; 0.2-0.7; 0.003) recipients. The predictors of new-onset SAIDs included presence of SAID multimorbidity (1.4; 1.1-1.7; < 0.001), mental health disorders (1.6; 1.3-1.9; < 0.001), and mixed race (2.2; 1.2-4.2; 0.010). Whereas, those with age >60 years (0.6; 0.4-0.8; 0.007), increasing vaccine doses (0.3; 0.2-0.5; < 0.001) and high/medium HDI countries (compared to very high HDI) (0.6; 0.4-0.8; 0.002) reported fewer new onset SAIDs.
Conclusion: This large case-series from the COVAD data set provides the first insights to new-onset SAID following COVID-19 vaccination in a global population. Due to disparity between patient and physician reporting, it is imperative to identify the correct diagnosis in individuals reporting new-onset SAID. Further research is therefore needed to facilitate counselling of individual risks and benefits, particularly if risk could be reduced by different COVID-19 vaccines schedules in predisposed individuals.
To cite this abstract in AMA style:
Shumnalieva R, Hannah J, ROY D, R N, Javaid M, Gonzalez D, Joshi M, Gracia-Ramos A, Cavagna L, Sen P, Day J, Saha S, Parodis I, Nikiphorou E, Kadam E, Knitza J, Aggarwal R, Agarwal V, Gupta L. New-Onset Systemic Autoimmune Diseases Following COVID-19 Vaccination – Results from the COVAD Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/new-onset-systemic-autoimmune-diseases-following-covid-19-vaccination-results-from-the-covad-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/new-onset-systemic-autoimmune-diseases-following-covid-19-vaccination-results-from-the-covad-study/