Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The autoinflammatory diseases are a group of disorders associated with dysregulation of the innate immune system. They are characterized by recurrent episodes of fever and systemic inflammation, typically presenting in early childhood. Since 1999, genetic causes have been discovered for several autoinflammatory diseases. Many patients with periodic fevers, however, are negative when tested for these mutations. Although the efficacy of interleukin-1 (IL-1) blockade in the known autoinflammatory diseases is established, this has not been systematically explored in the treatment of mutation-negative periodic fever syndromes. We describe our experience treating this heterogeneous group of children with the IL-1 receptor antagonist anakinra.
Methods: Records from patients enrolled in protocol 94-HG-0105 “Genetics and Pathophysiology of Familial Mediterranean Fever and Related Disorders” who were prescribed anakinra from 2009-2013 were reviewed. Patients were identified who had tested negative by commercially available methods for mutations known to cause autoinflammatory disease (MEFV, TNFRSF1A, NLRP3, and MVK). Results were confirmed by targeted sequencing. Medical records were reviewed for response to treatment based on patient-reported improvement in number or severity of flares. Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell (WBC) count, and ferritin values before and after treatment were obtained.
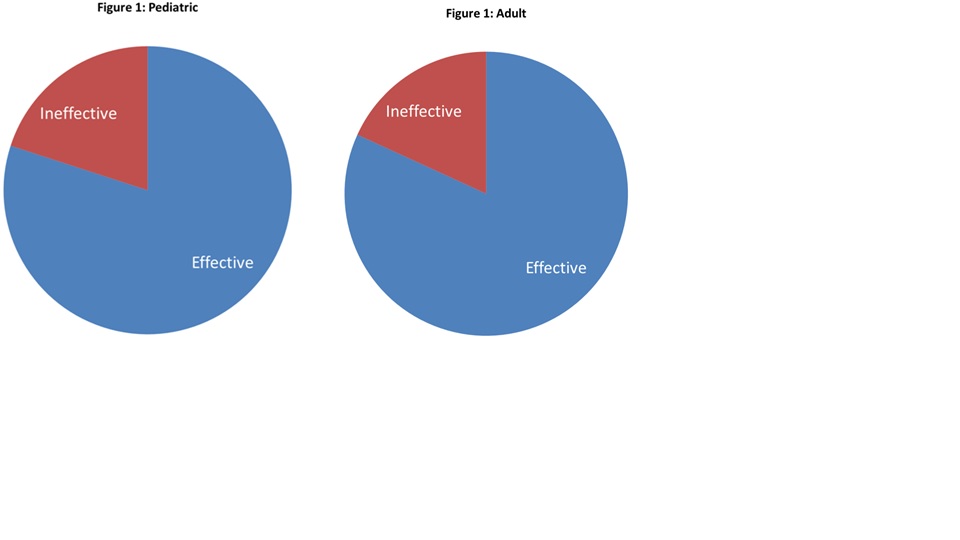
Results: Of 69 pediatric patients prescribed anakinra, 56 tested negative for mutations known to cause periodic fevers; longitudinal data were available in 35 children. 28 of 35 (80%) patients described a clinical response to anakinra (Figure 1). Decreases in erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell (WBC) count, and ferritin were statistically significant. Adverse reactions were minimal and consisted solely of injection site reactions. Of note, similar results were seen in the adult population, with 18 of 22 (82%) of patients describing a clinical response to anakinra, and statistically significant reduction in ESR and CRP.
Conclusion: Anakinra is safe and effective in the treatment of undifferentiated periodic fever syndromes.
Disclosure:
D. M. Schwartz,
None;
R. Wang,
None;
K. Barron,
None;
D. L. Kastner,
None;
A. K. Ombrello,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-interleukin-1-receptor-antagonist-anakinra-is-effective-in-the-treatment-of-undifferentiated-periodic-fever-syndromes/