Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Biologics are clinically efficacious in patients (pts) with axial spondyloarthritis (axSpA) including radiographic axSpA (r-axSpA). Limited data exist on the effect of biologics in slowing radiographic progression in pts with r-axSpA. Two-year data from MEASURE 1 showed low radiographic progression with secukinumab (SEC).1 We report data from SURPASS,2 the first head-to-head study in r-axSpA, that compared the effect of SEC vs adalimumab biosimilar (SDZ-ADL) on spinal radiographic progression.
Methods: In this phase IIIb study, bio-naïve pts with active r-axSpA with a Bath Ankylosing Spondylitis (AS) Disease Activity Index (BASDAI) ≥4, spinal pain score ≥4 (range 0–10), total back pain score ≥40 mm (range 0–100 mm), and with high-sensitivity C-reactive protein (hs-CRP) ≥5 mg/L or ≥1 syndesmophyte(s) on spinal radiograph were randomized (1:1:1) to SEC (150/300 mg; dose-blinded) or SDZ-ADL (40 mg; open label). Radiographs and MRIs were reviewed by 3 independent central readers blinded to treatment arm and chronology of images. Primary endpoint was the proportion of pts with no radiographic progression (change from baseline [CFB] in modified Stoke AS Spinal Score [mSASSS] ≤0.5; mean score of readers) on SEC vs SDZ-ADL at week (wk) 104 (superiority testing). Secondary endpoints included CFB-mSASSS by wk 104, proportion of pts with ≥1 syndesmophyte(s) at baseline (BSL) with no new syndesmophytes(s) at wk 104, CFB-MRI Berlin sacroiliac joint (SIJ) inflammation score, CFB-AS spine MRI-activity (ASspiMRI-a) Berlin modification score, and safety.
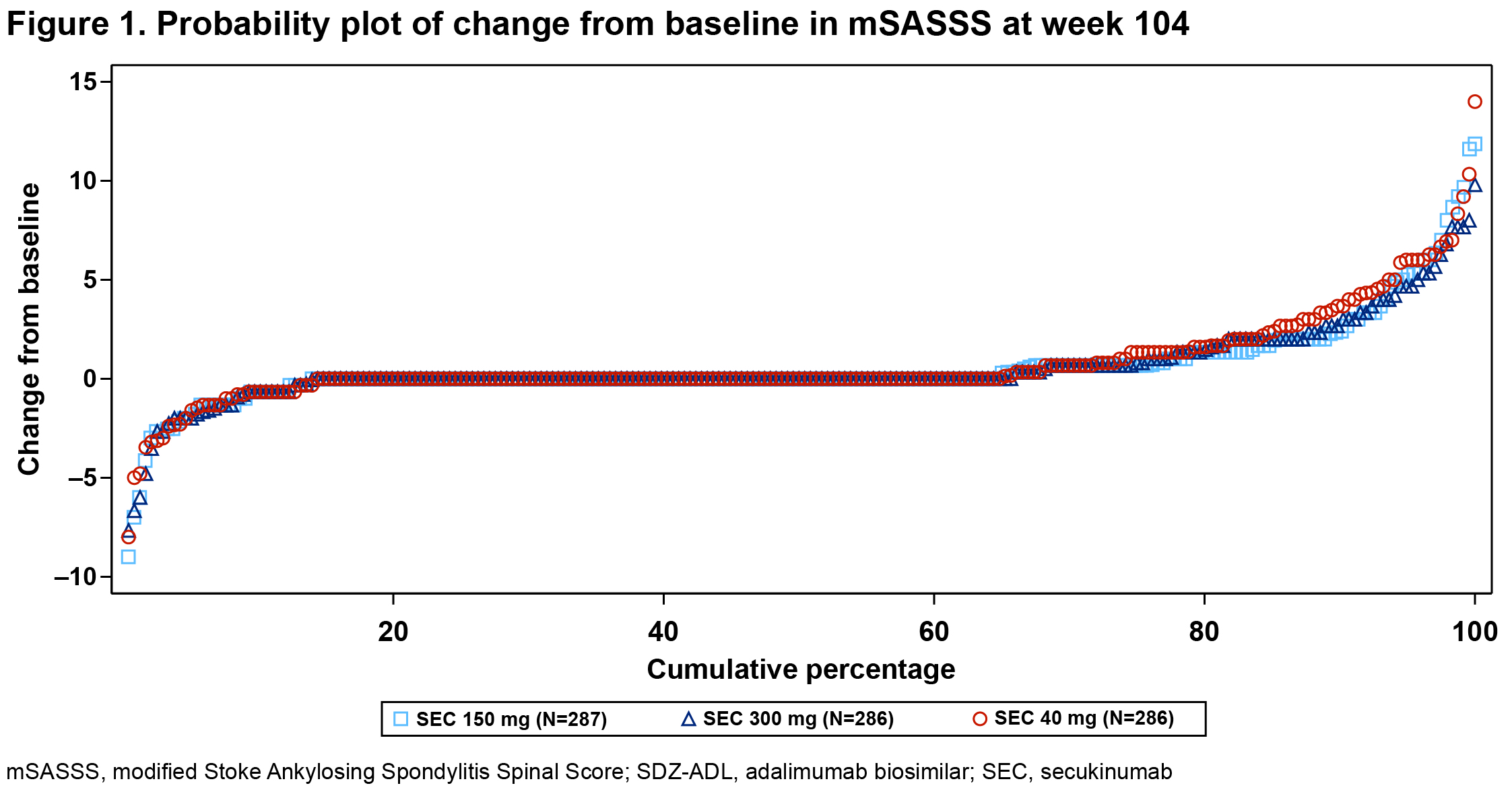
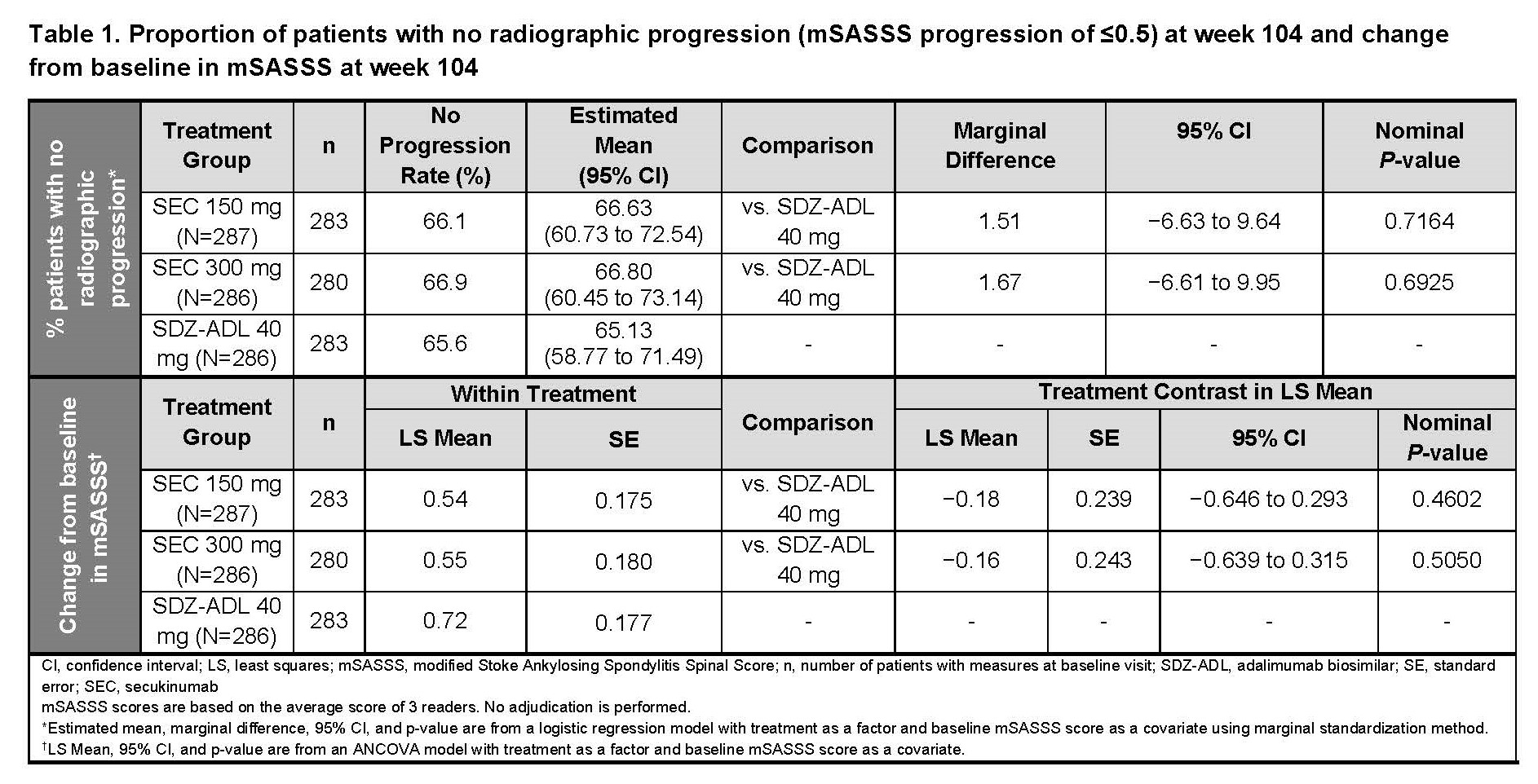
Results: Overall, 859 pts received SEC 150 mg (n=287), 300 mg (n=286), or SDZ-ADL (n=286). Pt demographics and BSL disease characteristics were balanced. With 78.5% male, mean age 42.1 years, mSASSS 16.6, BASDAI 7.1, hsCRP 20.4 mg/L, and 73% with ≥1 syndesmophyte(s), this population had a high risk of radiographic progression. Mean BSL MRI edema scores were 1.6 to 2.5 for SIJ and 2.6 to 3.4 for spine. At wk 104, the proportion of pts with no radiographic progression (CFB-mSASSS ≤0.5) was 66.1%, 66.9%, and 65.6% in SEC 150 mg, 300 mg, and SDZ-ADL arms, respectively (Table 1; P=ns, both SEC doses). Mean CFB-mSASSS was 0.54, 0.55, and 0.72 in SEC 150 mg, 300 mg, and SDZ-ADL arms, respectively (Table 1). Similar rates of CFB-mSASSS were observed across arms (Figure 1). Overall, 56.9%, 53.8%, and 53.3% of pts in SEC 150 mg, 300 mg, and SDZ-ADL arms, respectively, with a BSL ≥1 syndesmophyte(s) did not develop new syndesmophyte(s) by wk 104 (Table 2). At wk 16, mean (SE) CFB-MRI SIJ scores were −1.22 (0.14), −1.10 (0.14), and −1.51 (0.14), and mean CFB-MRI spine scores were −1.43 (0.14), −1.59 (0.15), −2.31 (0.15) in SEC 150 mg, 300 mg, and SDZ-ADL arms, respectively. Overall, 79.7%, 81.8%, and 84.2% pts had ≥1 adverse event (AE), and 14.0%, 10.2%, and 11.2% pts had serious AEs in SEC 150 mg, 300 mg, and SDZ-ADL arms, respectively.
Conclusion: Spinal radiographic progression over 2 years was low with no significant difference between SEC and SDZ-ADL arms. Safety was consistent with the well-established safety profiles of SEC and SDZ-ADL.
References
- Braun J et al. Ann Rheum Dis. 2017;76(6):1070-77
- Baraliakos X et al. Clin Drug Investig. 2020;40(3):269-78
To cite this abstract in AMA style:
Baraliakos X, Østergaard M, Poddubnyy D, van der Heijde D, Deodhar A, Machado P, Navarro-Compán V, Hermann K, Kishimoto M, Lee E, Gensler L, Kiltz U, Eigenmann M, Pertel P, Readie A, Richards H, Porter B, Braun J. Effect of Secukinumab versus Adalimumab Biosimilar on Radiographic Progression in Patients with Radiographic Axial Spondyloarthritis: A Randomized Phase IIIb Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effect-of-secukinumab-versus-adalimumab-biosimilar-on-radiographic-progression-in-patients-with-radiographic-axial-spondyloarthritis-a-randomized-phase-iiib-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-secukinumab-versus-adalimumab-biosimilar-on-radiographic-progression-in-patients-with-radiographic-axial-spondyloarthritis-a-randomized-phase-iiib-study/