Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Deucravacitinib (DEUC) is a novel, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor with a unique mechanism of action distinct from Janus kinase (JAK) 1/2/3 inhibitors. DEUC mediates signaling of key cytokines in PsA and psoriasis (PsO). DEUC was well tolerated and efficacious vs placebo (PBO) in a phase 2 trial in patients with PsA,1 and vs PBO or apremilast in 2 phase 3 PsO trials.2 The current analysis, conducted using data from the first 16 weeks (PBO-controlled phase) of the phase 2 PsA trial and pooled data from the 2 phase 3 PsO trials, assessed the effects of DEUC on multiple laboratory parameters, which are known to change on treatment with JAK 1/2/3 inhibitors.
Methods: The phase 2 double-blind PsA trial randomized patients (n = 203) 1:1:1 to PBO, DEUC 6 mg once daily (QD), or 12 mg QD. The phase 3 double-blind PsO trials, POETYK PSO-1 and POETYK PSO-2, randomized patients (n = 666 and 1020, respectively) 1:2:1 to PBO, DEUC 6 mg QD, or apremilast 30 mg twice daily. Changes from baseline in hematologic (neutrophils, lymphocytes, platelets, hemoglobin) and chemistry (cholesterol, triglycerides, alanine aminotransferase [ALT], aspartate aminotransferase [AST], and creatine phosphokinase [CPK]) parameters were evaluated. Shifts in Common Terminology Criteria for Adverse Events (CTCAE; version 5.0) severity grades ≥ 3 of laboratory abnormalities were assessed.
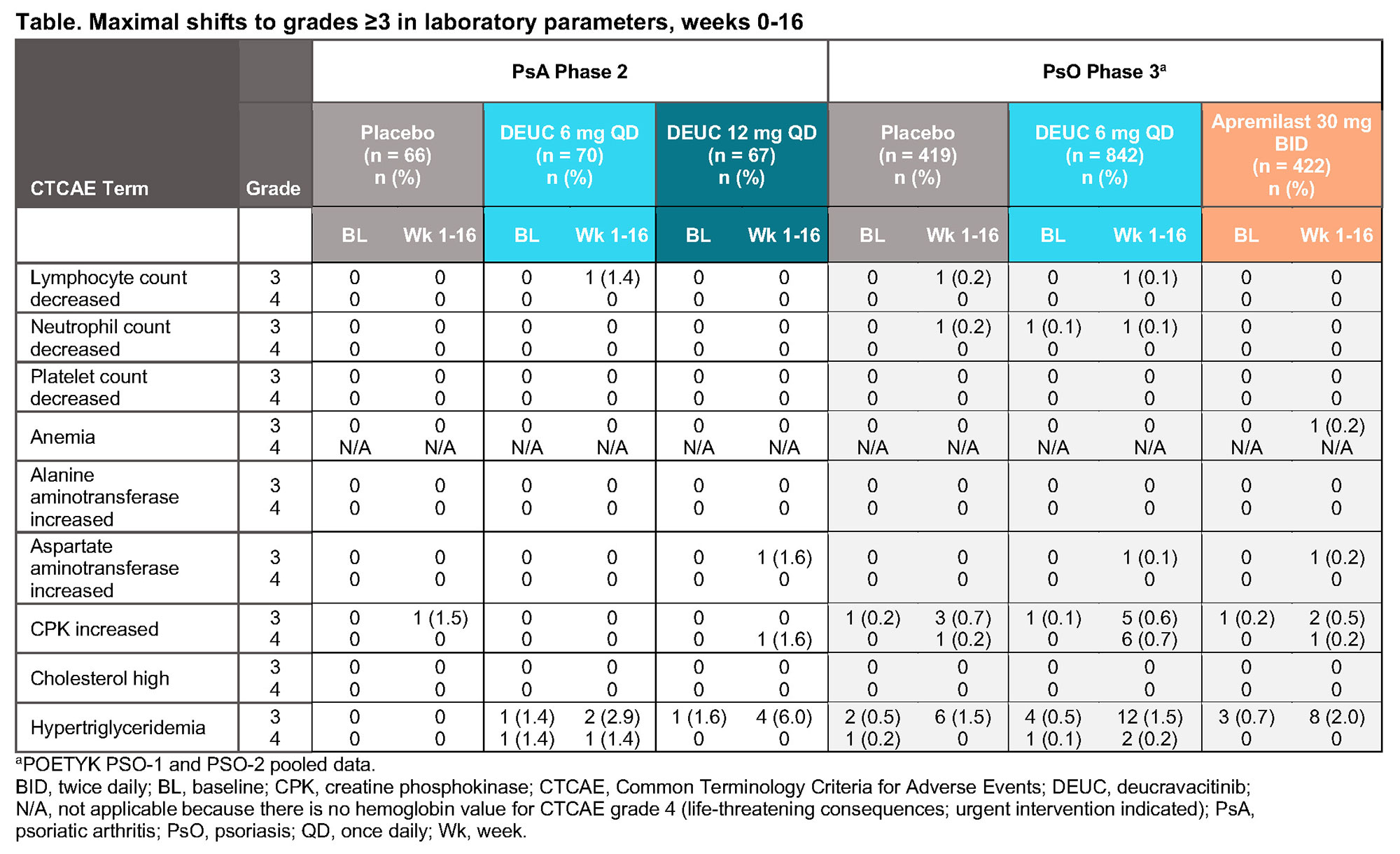
Results: In the PsA trial, 65% of patients were on concomitant conventional synthetic DMARDs (csDMARDs) and 54.7% of patients were on methotrexate. The vast majority of patients continued to have laboratory parameters within normal range throughout the 3 trials in PsO and PsA. No clinically meaningful changes from baseline were observed in any hematology, chemistry, or lipid laboratory parameters in patients treated with DEUC, PBO, or apremilast. Rates of CTCAE grades 3 and 4 were rare (≤ 1 patient) and similar across DEUC-, PBO-, and apremilast-treated patients for the following parameters: lymphocytes, neutrophils, platelets, hemoglobin, AST, ALT, and cholesterol (Table). Shifts to CTCAE grades ≥ 3 in triglycerides and CPK were infrequent and generally comparable across treatment arms.
Conclusion: DEUC treatment did not result in clinically meaningful mean changes from baseline in laboratory parameters over 16 weeks in the PBO-controlled portion of the phase 2 trial in PsA, despite two-thirds of patients receiving concomitant csDMARDs, and in 2 large phase 3 trials in PsO. Effects on hematological, hepatic, CPK, and cholesterol laboratory parameters characteristic of JAK 1/2/3 inhibitors were generally not observed over 16 weeks of DEUC exposure at doses up to 12 mg QD and in the presence of background csDMARDs.
References
1. Mease PJ, et al. Efficacy and safety of selective TYK2 inhibitor, deucravacitinib, in a phase II trial in psoriatic arthritis. Ann Rheum Dis. 2022;81:815-822.
2. Armstrong A, et al. Presented at American Academy of Dermatology Virtual Meeting Experience 2021; April 23-25, 2021.
To cite this abstract in AMA style:
Fleischmann R, Thaci D, Gooderham M, Strober B, Korman N, Banerjee S, Lehman T, Nowak M, Sreih A, Morita A, Mease P. Safety of Deucravacitinib, an Oral, Selective Tyrosine Kinase 2 Inhibitor: As Assessed by Laboratory Parameters – Results from a Phase 2 Trial in Psoriatic Arthritis and 2 Phase 3 Trials in Psoriasis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/safety-of-deucravacitinib-an-oral-selective-tyrosine-kinase-2-inhibitor-as-assessed-by-laboratory-parameters-results-from-a-phase-2-trial-in-psoriatic-arthritis-and-2-phase-3-trials-in-ps/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-deucravacitinib-an-oral-selective-tyrosine-kinase-2-inhibitor-as-assessed-by-laboratory-parameters-results-from-a-phase-2-trial-in-psoriatic-arthritis-and-2-phase-3-trials-in-ps/