Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Risankizumab (RZB) is a monoclonal antibody that specifically inhibits interleukin 23.
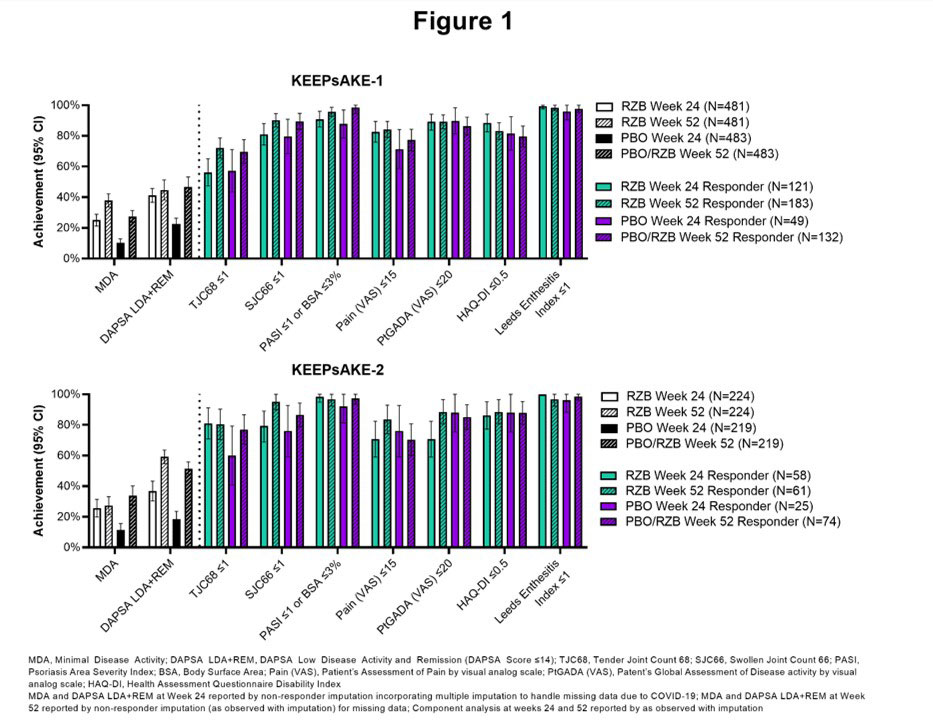
Methods: KEEPsAKE-1 and -2, double-blind, phase 3 trials, evaluated the efficacy of RZB versus placebo (PBO) for the treatment of adult patients with active psoriatic arthritis (PsA). Patients were randomized (1:1) to receive subcutaneous RZB 150 mg or PBO at weeks 0, 4, and 16. The open label extension began at Week 24 with all patients receiving RZB 150 mg every 12 weeks thereafter. Achievement of Minimal Disease Activity (MDA), its components, and achievement of Disease Activity in PsA Low Disease Activity and Remission (DAPSA LDA+REM, [DAPSA score ≤14]) are reported using non-responder imputation.
Results: MDA achievement at Week 52 in KEEPsAKE-1 was 37.9% for patients originally randomized to RZB and 27.4% for patients originally randomized to PBO. In KEEPsAKE-2, MDA achievement was 27.2% and 33.8% for patients originally randomized to RZB and PBO, respectively. Achievement of MDA and its components are presented in Figure 1. In KEEPsAKE-1, at Week 52 59.2% of patients originally randomized to RZB and 51.4% of patients originally randomized to PBO achieved DAPSA LDA+REM. At Week 52 in KEEPsAKE-2, DAPSA LDA+REM was achieved by 44.6% of patients originally randomized to RZB and 46.6% of patients originally randomized to PBO (Figure 1).
Conclusion: Patients treated with RZB demonstrate achievement of MDA, its components, and DAPSA LDA+REM at Weeks 24 and 52.
To cite this abstract in AMA style:
Merola J, McInnes I, Kavanaugh A, Nash P, Xue Z, Stakias V, Eldred A, Ciecinski S, Douglas K, Coates L. Effects of Treatment with Risankizumab on Minimal Disease Activity and Disease Activity in Psoriatic Arthritis: An Analysis of the KEEPsAKE-1 and -2 Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effects-of-treatment-with-risankizumab-on-minimal-disease-activity-and-disease-activity-in-psoriatic-arthritis-an-analysis-of-the-keepsake-1-and-2-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effects-of-treatment-with-risankizumab-on-minimal-disease-activity-and-disease-activity-in-psoriatic-arthritis-an-analysis-of-the-keepsake-1-and-2-trials/