Session Information
Date: Monday, November 14, 2022
Title: Abstracts: SLE – Treatment
Session Type: Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: Hydroxychloroquine (HCQ) is the backbone of SLE therapy, due to its benefits on increasing survival and decreasing lupus flares. In 2011, ophthalmology guidelines recommended limiting HCQ dosing to ≤6.5mg/kg of ideal body weight to reduce the risk of retinopathy; in 2016 updated guidelines recommended using ≤5mg/kg of actual body weight. For many patients, these guidelines require a dose lower than 400mg/day, which had been frequently used to treat SLE. However, the impact of the lower HCQ dosing guidelines on the risk of SLE hospitalizations is unknown. We sought to determine the impact of HCQ dose on the risk of hospitalizations for SLE flares.
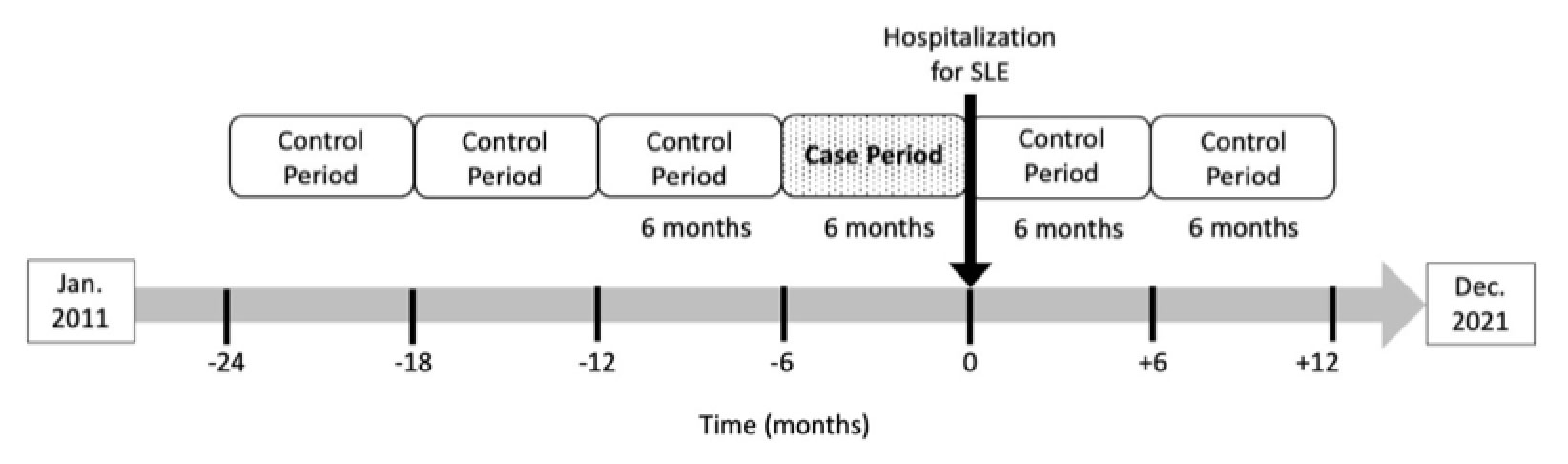
Methods: We conducted a case-crossover study within the Mass General Brigham SLE cohort, including two US academic medical centers. We identified patients with SLE from an electronic health record-based algorithm (positive predictive value >90%, Jorge A. Semin Arthritis Rheum 2019) who had ≥1 visit for SLE and were prescribed HCQ between January 2011-December 2021. Within this cohort, we identified patients who had a hospitalization during this period with SLE as the primary discharge diagnosis code. We reviewed each hospitalization record and excluded those for non-SLE indications (e.g., kidney transplant, infection without a concomitant SLE flare). Patients with ≥1 hospitalization for SLE flare while using HCQ were included in the case-crossover study. Case periods were defined as 6 months prior to a SLE hospitalization. Control periods were defined as non-overlapping 6-month periods without a SLE hospitalization. Periods without HCQ use were excluded. Patients could have up to 3 case periods and 3 control periods (Figure 1). The exposures of interest were the average weight-based HCQ dose, categorized as ≤5 or >5mg/kg/day, and the average non-weight-based HCQ dose, categorized as < 400 or 400mg/day, assessed during each 6-month case or control period. Odds ratios (OR) were calculated using conditional logistic regression and adjusted for SLEDAI, glucocorticoid use and other immunosuppressant use assessed at most recent outpatient visit prior to each 6-month period.
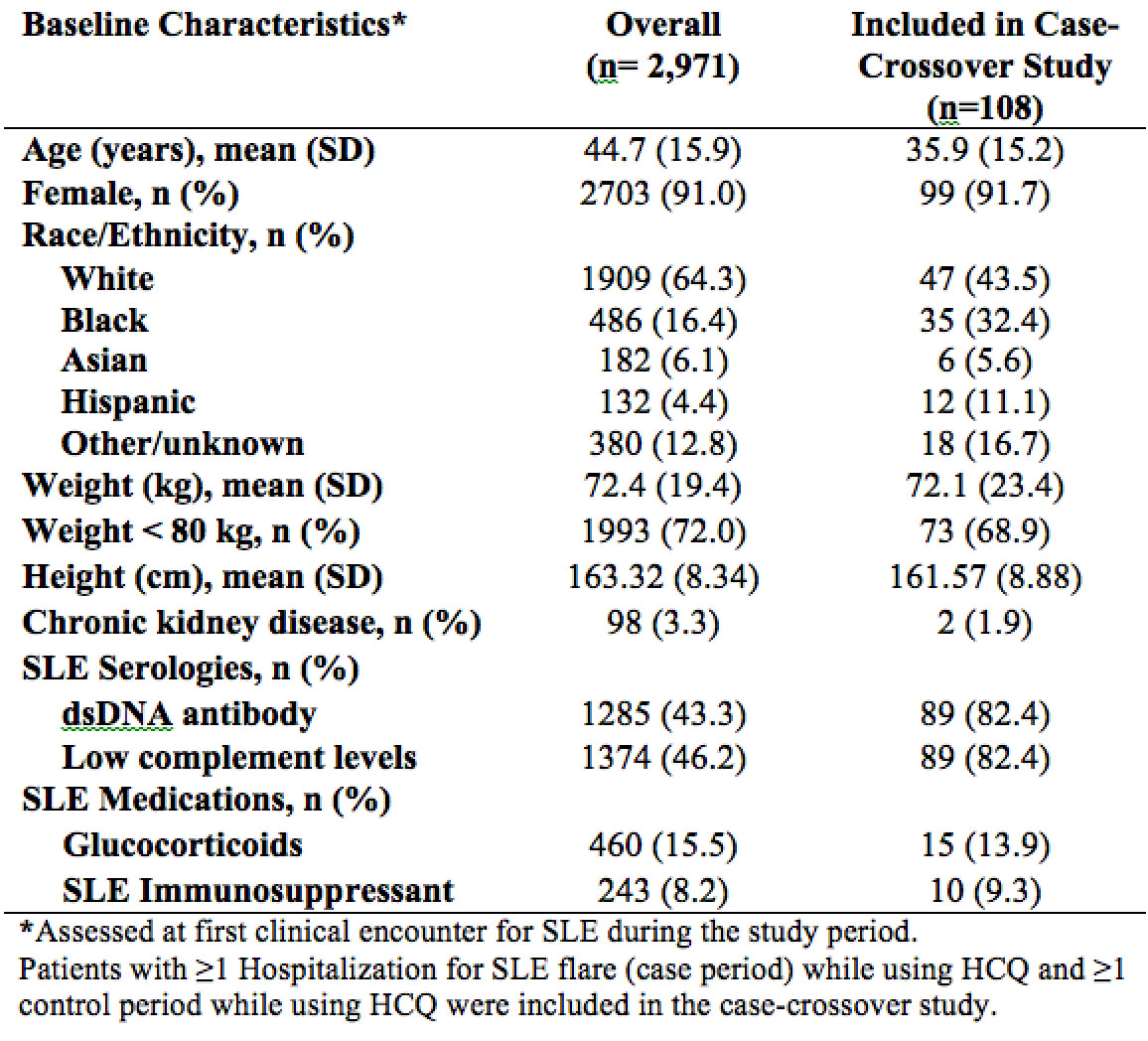
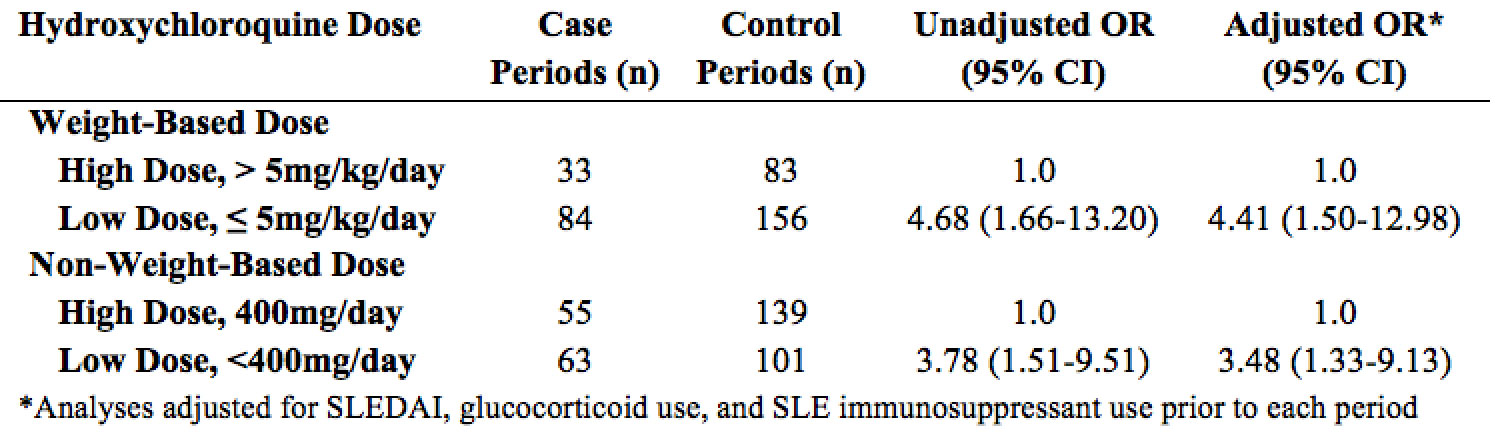
Results: Of 2,971 patients with SLE who used HCQ, 576 had ≥1 hospitalization with primary discharge diagnosis of SLE. Of these, 108 were hospitalized for a SLE flare while using HCQ and had ≥1 control period with HCQ use during the study period (Table 1). The average age of the hospitalized group was 36 years; 92% were female. 43.5% were white and 32.4% were black. Low dose HCQ by weight-based dose (≤5 vs 5 mg/kg/day) and by non-weight-based dose (< 400 vs 400 mg/day) were both associated with increased hospitalizations for SLE, adjusted OR 4.41 (95% CI 1.50-12.98) and 3.48 (95% CI 1.33-9.13) respectively (Table 2).
Conclusion: SLE patients treated with lower doses of HCQ had an increased risk of hospitalizations for SLE flares. Study limitations include incomplete information on medication adherence and reasons for using lower dose HCQ. Nonetheless, these results indicate the need to reassess the optimal dosing of HCQ for patients with SLE. Although the long-term risk of HCQ-induced retinopathy must be acknowledged, this must be balanced with the short-term and cumulative risks of increased SLE flares.
To cite this abstract in AMA style:
Nestor J, Mancini C, Zhou B, Zhang Y, Costenbader K, Choi H, Jorge A. Hydroxychloroquine Dosing Less Than 5 Mg/kg/day Leads to Increased Hospitalizations for Systemic Lupus Erythematosus Flares [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-dosing-less-than-5-mg-kg-day-leads-to-increased-hospitalizations-for-systemic-lupus-erythematosus-flares/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-dosing-less-than-5-mg-kg-day-leads-to-increased-hospitalizations-for-systemic-lupus-erythematosus-flares/