Session Information
Date: Sunday, November 13, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Interleukin-6 receptor (IL-6R) inhibition has been shown to be effective in giant cell arteritis but data are limited in polymyalgia rheumatica (PMR). We conducted a retrospective study to evaluate effectiveness of IL-6R inhibitors (IL-6Ri; sarilumab or tocilizumab) in 3rd-line and 2nd-line treatment of glucocorticoid (GC)-refractory PMR patients compared to conventional immunomodulatory (cIM) therapy.
Methods: Adults with PMR, defined as 1 inpatient or 2 outpatient claims with a PMR diagnosis ≥30 days apart, were identified from national fee-for-service Medicare (100% sample) data between 3/29/2016- to 6/30/2020. Included were those who initiated IL-6Ri or cIM as 2nd-line and 3rd-line therapy for PMR and had continuous enrollment for 180 days prior to therapy initiation (baseline). For 3rd-line cohort, the index date was date of switch from cIM to IL-6Ri for the IL-6Ri group and switch from one cIM to another cIM for the cIM group. For 2nd-line cohort, the index date was the date of initiation of IL-6Ri with no prior cIM for the IL-6Ri group and new use of cIM for the cIM group. A 180-day washout period for the index drug was applied to all cohorts. Patients were on GC on index date (≤25mg/day) and excluded if they had evidence of seropositive rheumatoid arthritis, other inflammatory arthritis or connective tissue disease, multiple sclerosis, or malignancy. Patients on IL-6Ri and cIM were matched 1:1 on age, sex, and GC dose category, and propensity score matching was used to adjust for potential confounders.
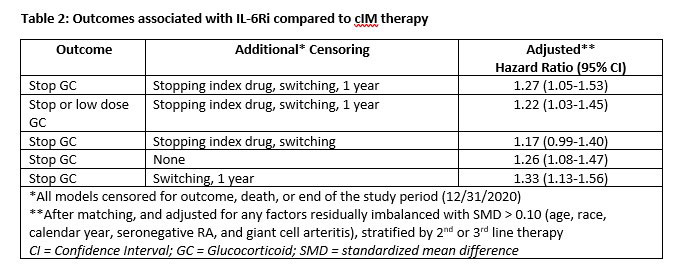
Outcomes assessed using adjusted Cox proportional hazards models included GC discontinuation; GC discontinuation or low dose GC (< 2mg/day); and non-persistence (discontinuation of IL-6Ri or cIM, or switch) in the 2nd-line cohort, adjusting for residually imbalanced factors after matching. Censoring occurred at 1 year, death, 60 days before end of enrollment, or addition of comparator PMR therapy with additional censoring applied in sensitivity analysis for drug discontinuation and/or switching.
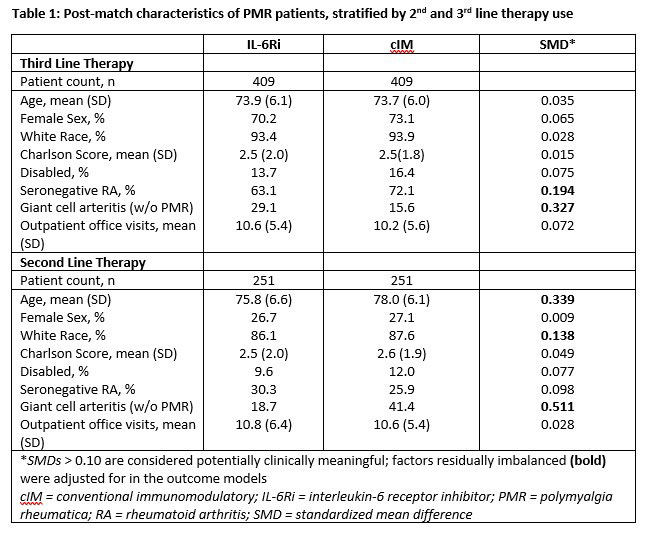
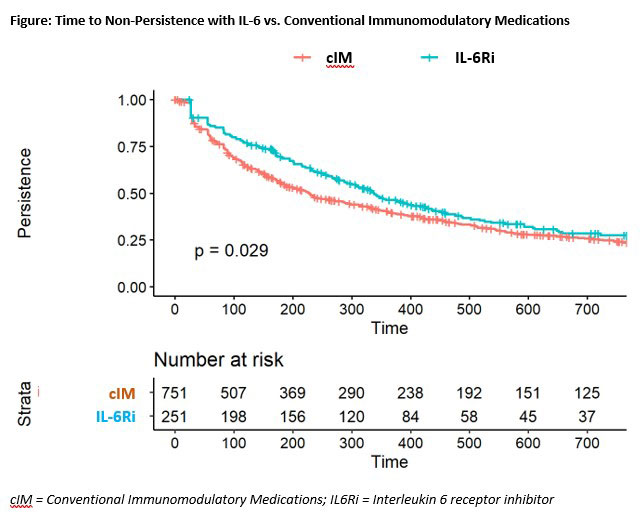
Results: After matching, 409 3rd-line and 251 2nd-line patients were included in the analysis. Post-match, patient characteristics were generally well balanced between cohorts, with small differences between exposure groups (Table 1). Median time from first PMR diagnosis to start of follow-up was approximately 1.75 years (3rd-line) and 0.90 years (2nd-line) and similar between groups. After matching and adjustment, patients receiving IL-6Ri were more likely to achieve GC discontinuation or low dose GC, compared to cIM patients (Table 2). Time to discontinuation (Figure) was significantly greater for IL-6Ri vs. cIM patients (p=0.029).
Conclusion: IL-6Ri therapy was more effective as a steroid sparing agent compared to cIM therapy for adult patients with PMR.
To cite this abstract in AMA style:
Curtis J, Ford K, Fiore S, Isaman D, Araujo L, Petruski-Ivleva N, Xie F. Effectiveness of Interleukin-6 Receptor Inhibitors for Polymyalgia Rheumatica [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effectiveness-of-interleukin-6-receptor-inhibitors-for-polymyalgia-rheumatica/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-of-interleukin-6-receptor-inhibitors-for-polymyalgia-rheumatica/