Session Information
Date: Saturday, November 12, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Giant Cell Arteritis (GCA) may be refractory to standard treatment with glucocorticoids and conventional and/or biologic disease-modifying antirheumatic drugs (cDMARDs/bDMARDs). The JAK/STAT signaling pathway is involved in GCA; thus, JAK inhibitors (JAKINIBs) may be useful in this disease. Baricitinib (BARI) seems useful for GCA in a clinical open-label pilot study (Koster MJ, et al. Ann Rheum Dis. 2022;81:861-8). However, most patients had not previously received DMARDs, and none had received tocilizumab the only biologic drug approved in GCA. We assess the effectiveness and safety of JAKINIBs in refractory GCA to cDMARDs and/or bDMARDs.
Methods: National multicenter study of clinical practice and literature review of JAKINIBs-treated GCA. For the literature review, a search of PubMed, Embase and the Cochrane library was conducted from inception to 15 May 2022. A comparative study between the previous pilot study (Koster MJ, et al. Ann Rheum Dis. 2022 Jun;81(6):861-86) and this series of real clinical practice was performed.
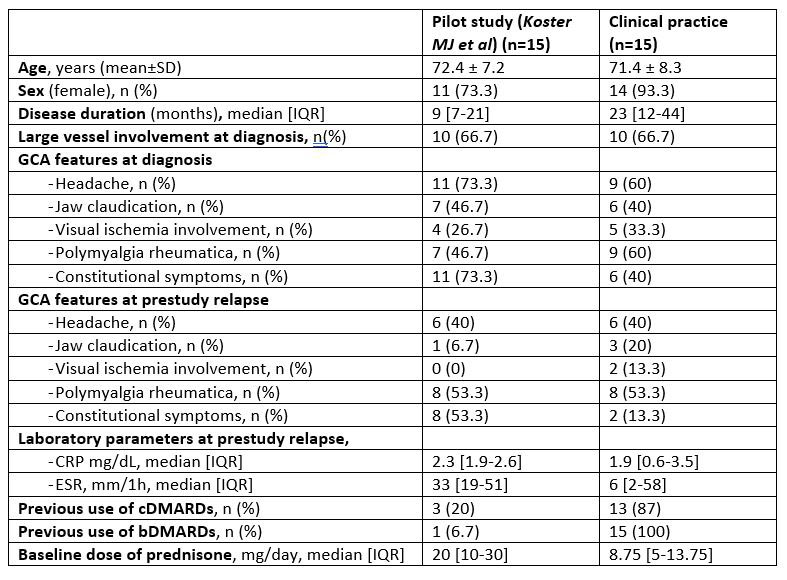
Results: We included 15 patients (14 ,93.3% female) with a mean age of 71.4±8.3 years. They were refractory to methotrexate (MTX) (n=12; 80%), IL-6 receptor antagonists (n=12; 80%), abatacept (ABA) (n=3; 25%), and ustekinumab (USTE) (n=1; 7%). The following JAKINIBs were firstly used, Baricitinib (BARI) (n=7) (4 mg/24 h; n=4) (2 mg/24h; n=3), tofacitinib (TOFA) (n= 5) (5 mg/12h), and upadacitinib (UPA) (n=3) (15 mg/24h). Patients of clinical practice had longer disease duration, received DMARDs more commonly and were on a lower baseline dose of prednisone than those in the open label study (Table 1).
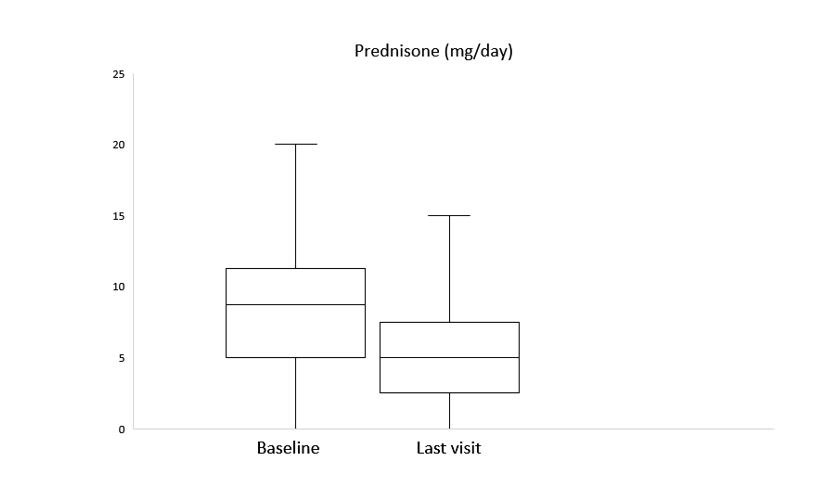
After a median follow-up of 3 [1-11] months, clinical improvement was observed in 6 (85.7%), 3 (60%) and 2 (66.7%) with BARI, TOFA and UPA, respectively. A reduction of prednisone dose was achieved as well (Figure). Elevation of liver function tests was observed in one patient with BARI who was switched to UPA maintaining clinical improvement. No other severe adverse events were reported. Another 2 patients were switched to a second JAKINIB: one patient from UPA to BARI due to inefficacy and another one from TOFA to FILGO due to warning in older age and cardiovascular comorbidities, pending clinical response evaluation due to recent change.
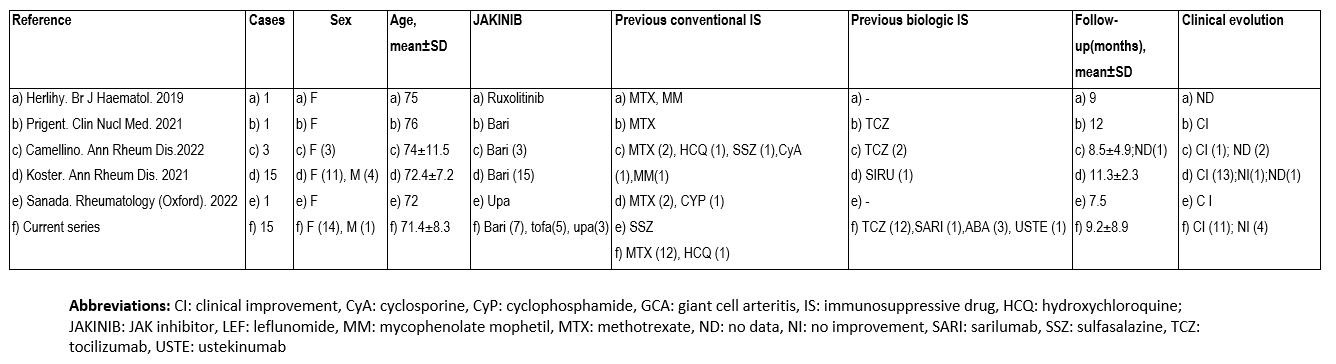
In the literature review we found 21 patients with GCA (17 women, 74.2±.1.7 years). BARI was the most used (n=26, 72.2%). Most patients improved with JAKINIBs (Table 2).
Conclusion: JAKINIBs appear to be an effective and safety therapy in refractory GCA to cDMARDs and/or bDMARDs.
To cite this abstract in AMA style:
Prieto-Peña D, Loricera J, Romero Yuste S, Riveros-Frutos A, Narváez F, De Miguel E, Emperiale V, Becerra-Fernández E, Castañeda S, Labrador E, Maiz O, González-Gay M, Blanco R. JAKINIB in Refractory Giant Cell Arteritis. National Multicenter Study of 15 Cases and Literature Review [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/jakinib-in-refractory-giant-cell-arteritis-national-multicenter-study-of-15-cases-and-literature-review/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/jakinib-in-refractory-giant-cell-arteritis-national-multicenter-study-of-15-cases-and-literature-review/