Session Information
Date: Saturday, November 12, 2022
Title: Vasculitis – Non-ANCA-Associated and Related Disorders Poster I: Giant Cell Arteritis
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Hemoglobin A1c (HbA1c) is used clinically to estimate patients’ average blood glucose over the preceding few months and is affected by glycemic as well as non-glycemic factors (e.g., red blood cell [RBC] age distribution, hemoglobin variants, iron deficiency anemia, pregnancy, and ethnicity). The Trial of Tocilizumab in Giant Cell Arteritis (GiACTA) randomized patients with giant cell arteritis (GCA) to either prednisone alone (GC-only) or prednisone in combination with tocilizumab (tocilizumab/GC). The GiACTA protocol required GC tapering in all treatment groups, with a more rapid taper in those in the tocilizumab/GC group, and HbA1c and RBC counts were measured regularly during the one-year follow-up period. Although the association between the initiation of glucocorticoids (GCs) and the development of hyperglycemia is well-known, the longitudinal effects of tocilizumab or tapering GCs on HbA1c have not been studied. We assessed the effects of decreasing daily GC dose, randomization to tocilizumab/GC, and RBC count on longitudinal changes in HbA1c.
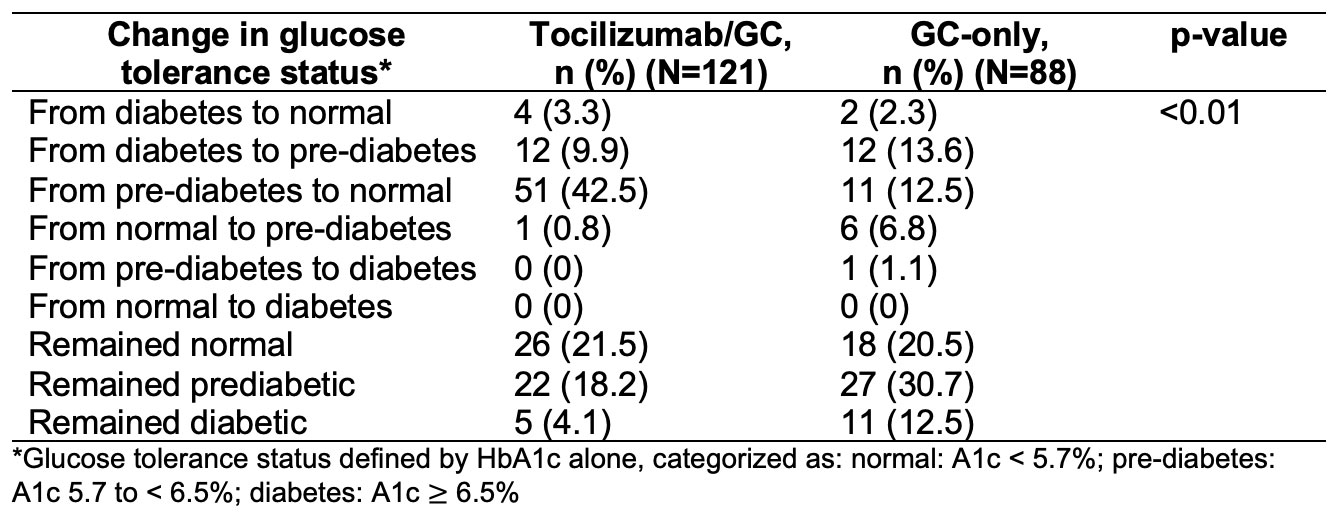
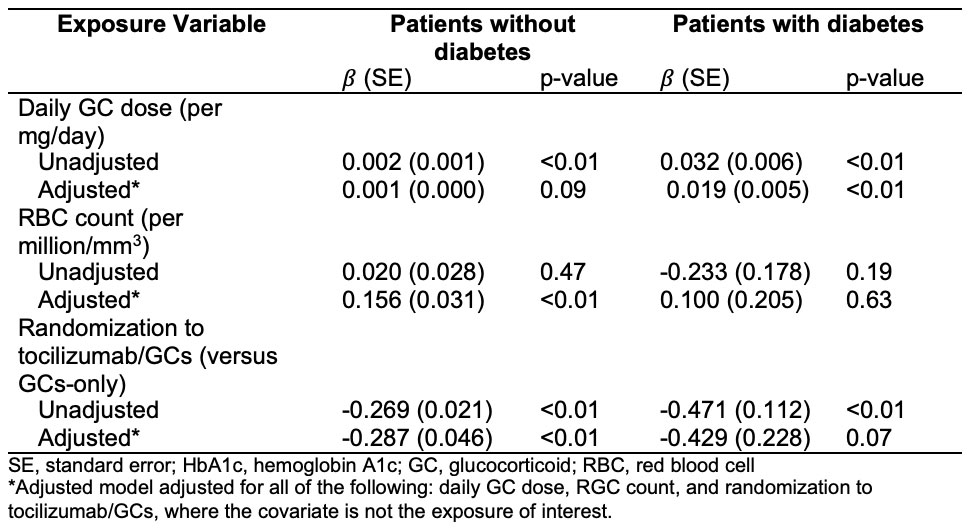
Methods: We analyzed patients with complete data from the GiACTA trial to investigate the impact of both glycemic and non-glycemic factors on changes in HbA1c over the 52-week trial. We used Fisher’s exact test to compare the changes in HbA1c category (normal, pre-diabetes, or diabetes) from week 0 to 52 between treatment groups. We used a multivariable mixed-effects model to evaluate associations of HbA1c with daily GC dose, randomization to tocilizumab/GC, and red blood cell count over 52 weeks in patients with and without baseline diabetes, defined as either a pre-existing diagnosis of diabetes, HbA1c ≥6.5 at baseline, or medication at baseline for the management of diabetes.
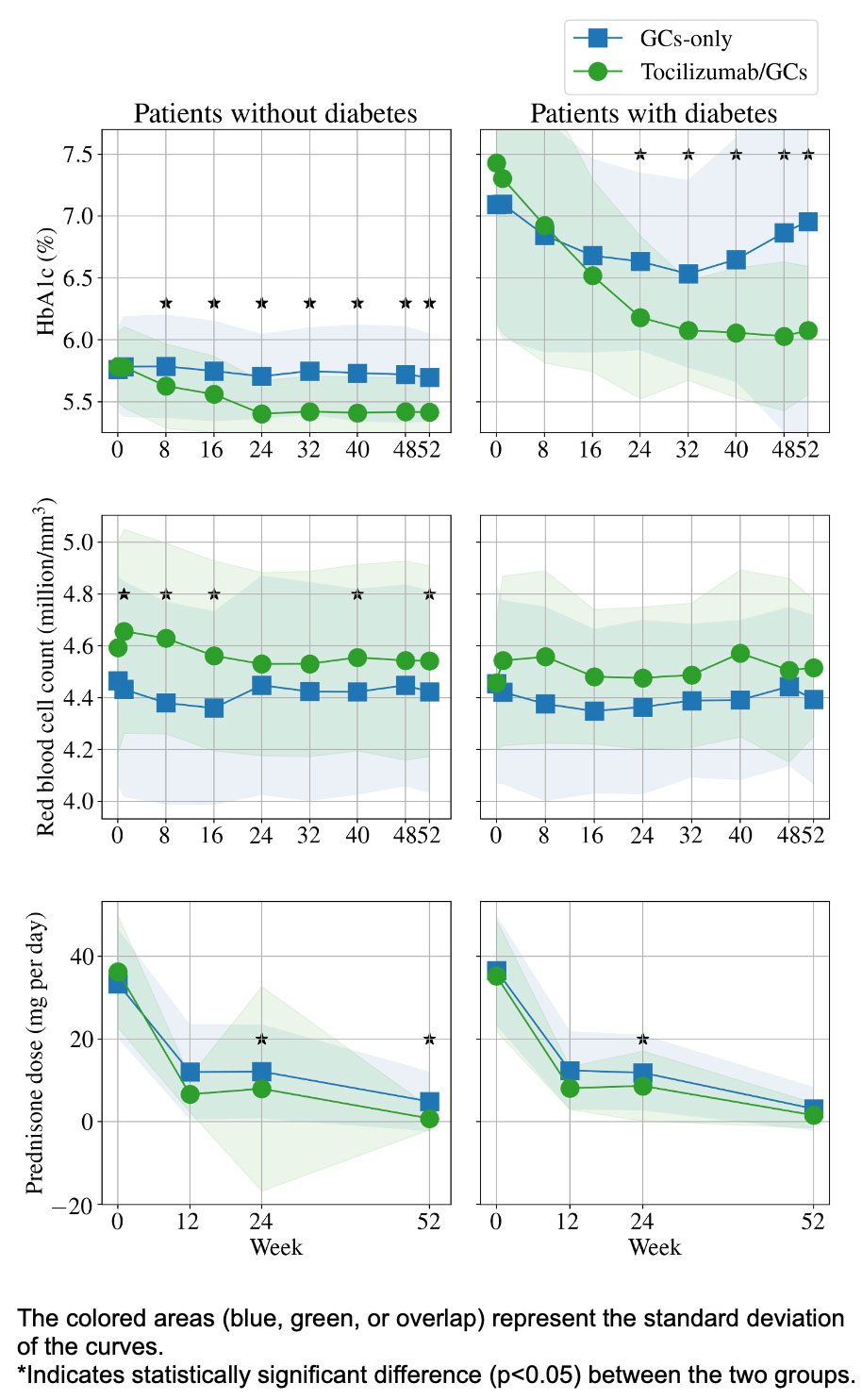
Results: In 209 patients (mean age 69 years; 76% female), the median HbA1c decreased by 0.50% (p< 0.01) in the tocilizumab/GC group and by 0.10% (p=0.01) in the GC-only group (Figure). Randomization to tocilizumab/GC was independently associated with lower HbA1c (β=-0.287% in those without diabetes, p< 0.01; β=-0.429% in those with diabetes, p=0.07). These changes had a sizable impact on glucose tolerance classification: 42.5% in the tocilizumab/GC group improved from pre-diabetes status to normal, compared with only 12.5% of patients treated with GC alone (Table 1). Daily GC dose was associated with HbA1c in patients with diabetes (β=0.019% per mg GC, p< 0.01) but had a non-significant association in patients without diabetes (Table 2).
Conclusion: Tocilizumab treatment was associated with a substantial reduction in HbA1c, independent of GC exposure, which may be achieved through a combination of glycemic and non-glycemic effects. GC tapering is associated with a reduction in HbA1c in those with diabetes at baseline, underscoring the importance of minimizing cumulative GC exposure, especially in patients with impaired glucose tolerance. Further studies involving intensive glucose monitoring may help to better understand the mechanism of the glycemic and/or non-glycemic effects of tocilizumab on HbA1c and guide optimal use and interpretation of HbA1c levels.
To cite this abstract in AMA style:
Patel N, Tozzo V, Higgins J, Stone J. The Effects of Daily Prednisone and Tocilizumab on Hemoglobin A1c During the Treatment of Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/the-effects-of-daily-prednisone-and-tocilizumab-on-hemoglobin-a1c-during-the-treatment-of-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effects-of-daily-prednisone-and-tocilizumab-on-hemoglobin-a1c-during-the-treatment-of-giant-cell-arteritis/