Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Ixekizumab (IXE) is a high-affinity monoclonal antibody that selectively targets IL-17A approved for the treatment of psoriasis, psoriatic arthritis (PsA), active ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation. We report a summary of the safety outcomes with over 2000 patient-years (PY) of exposure up to 3 years in patients with PsA and axSpA.
Methods: Long-term safety of IXE was assessed from 8 randomized trials. Treatment-emergent adverse events (TEAEs) adjusted incidence rates (IRs) per 100 PY within 1-year time periods through 19 March 2021 were calculated for all patients treated with ≥1 dose of IXE. Safety outcomes included TEAEs, serious AEs (SAEs), discontinuations due to AEs, deaths, and selected safety topics of interest. Major adverse cerebro-cardiovascular event (MACE) and inflammatory bowel disease (IBD) reported cases were adjudicated.
Results: A total of 1401 patients with PsA and 932 patients with axSpA with a cumulative IXE exposure of 2247.7 PY for PsA and 2096.2 PY for axSpA were included in this analysis (Table). The IRs per 100 PY for any TEAE were 50.3 for PsA and 38.1 for axSpA. Serious AEs were reported by 134 patients with PsA (IR=6.0), and 101 patients with axSpA (IR=4.8). A total of 9 deaths was reported, including 6 in PsA (IR= 0.3) and 3 in axSpA (IR=0.1). The IRs per 100 PY of discontinuation from the study drug due to AE were 5.1 (PsA) and 3.1 (axSpA). IRs of serious infections were low (PsA: IR= 1.2, axSpA: IR=1.1). IRs of opportunistic infections (PsA: IR= 1.8, axSpA: IR=0.8) and Candida infections (PsA: IR= 2.0, axSpA: IR=1.2) were low. There were no confirmed cases of reactivation of tuberculosis. Injection site reactions occurred with IRs of 11.6 (PsA) and 7.4 (axSpA). The IRs for allergic/hypersensitivity reactions were 4.5 (PsA) and 4.2 (axSpA). No confirmed events of anaphylaxis were reported. Across indications, IRs were low for cytopenia (≤2.5), malignancies (≤0.7), MACE (≤0.5), depression (≤1.6), and iridocyclitis (≤2.8). Per external adjudication, 20 patients had confirmed IBD (including 3 patients with PsA and 17 with axSpA) of which 1 was confirmed as ulcerative colitis for PsA (IR=0.0) and 10 for axSpA (IR=0.5); 2 events were confirmed as Crohn’s disease for PsA (IR=0.1) and 7 for axSpA (IR=0.3). Across safety topics, IRs were decreased or remained constant over time (Figures 1 and 2).
Conclusion: In this updated analysis with 2247.7 PY for PsA and 2096.2 PY for axSpA, IXE maintained a safety profile consistent to that previously reported,1-3 with no new or unexpected safety events through exposure up to 3 years.
References:
1Mease P et al. Arthritis Care Res (Hoboken) 2019;71(3):367-78
2Combe B et al. Arthritis Res Ther 2020;22(1):14
3Genovese MC et al. Rheumatology (Oxford) 2020;59(12):3834-44
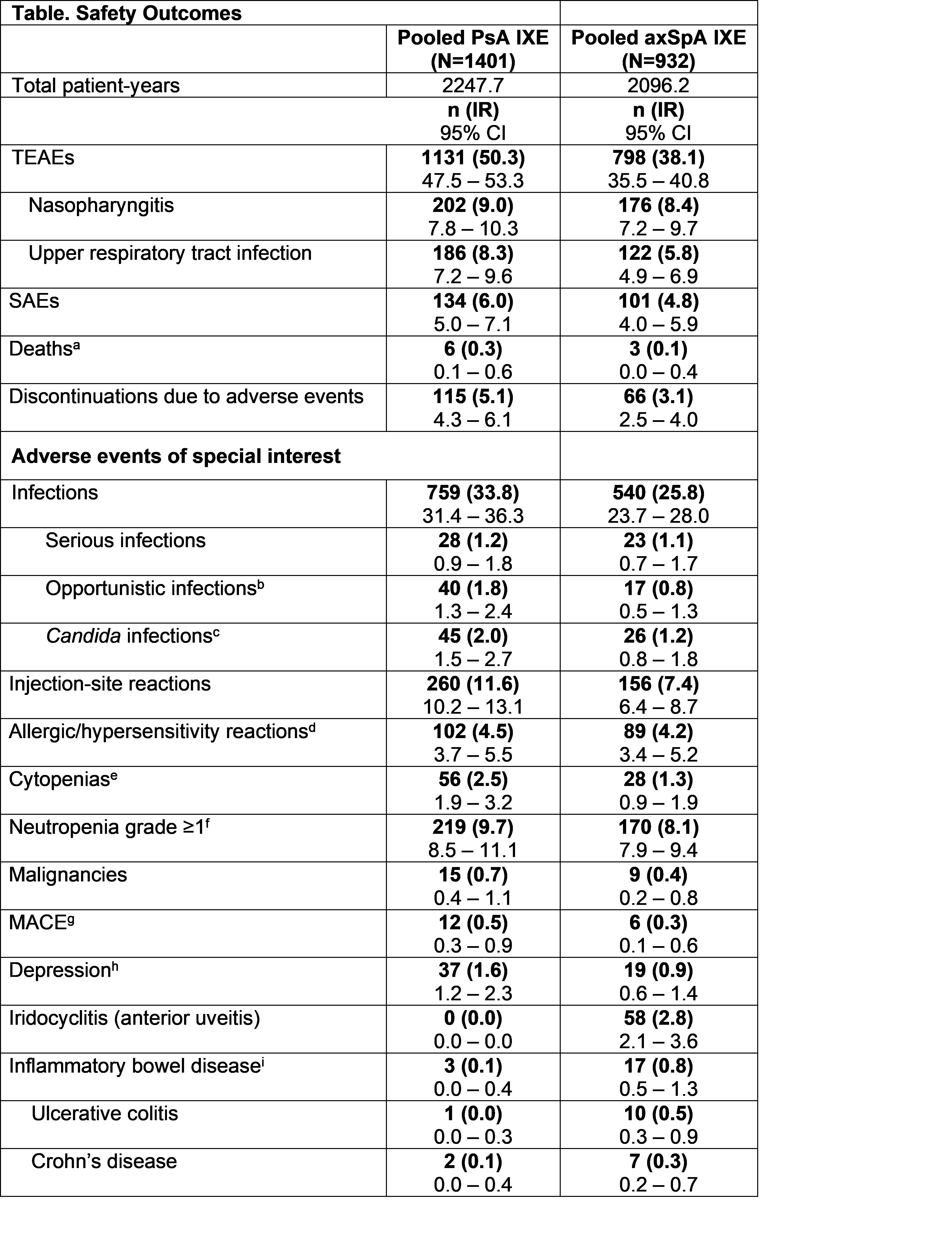 axSpA, axial spondyloarthritis; IR, incidence rate; IXE, ixekizumab; MACE, major adverse cerebro-cardiovascular events; n, number of patients in each category; PsA, psoriatic arthritis; SAEs, serious adverse events; SMQ, Standardised MedDRA Queries; TEAEs, Treatment-emergent adverse events. Adverse event (AE) terms were derived from MedDRA v23.1. aThe 6 reported deaths in the PsA population were due to cardiovascular event (n=2), metastatic renal cell carcinoma (n=1), cerebrovascular accident (n=1), pneumonia (n=1), and drowning (n=1). In the axSpA population, the causes of deaths were suicide (n=1), sepsis (n=1), and murder (n=1). bOpportunistic infections included esophagus candidiasis, oral candidiasis, hepatitis B reactivation, and herpes zoster. cSome Candida infections cases were also considered as opportunistic infections. All cases of Candida infection were localized. In PsA program, most cases were mild (34/45, IR=1.5 per 100 patient-years) to moderate (10/45, IR=0.4 per 100 patient-years) in severity, except 1 case of esophagus candidiasis considered severe. The patient who reported severe esophagus candidiasis did not discontinue the study drug due to this AE. In axSpA program, all cases were mild (16/26, IR=0.8 per 100 patient-years) or moderate (10/26, IR=0.5 per 100 patient-years) in severity. Across indications, no cases of systemic candidiasis were reported. dNo cases of anaphylaxis were confirmed after medical reviews. eBroad, according to SMQ classification. fBased on neutrophils count lab results. Neutropenia grade ≥3, n (IR) [95%CI] PsA= 8 (0.4) [0.2, 0.7], axSpA= 4 (0.2) [0.1, 0.5]. gThe data represents events confirmed by adjudication. hBroad, according to SMQ or sub-SMQ classification. iThe data represents confirmed cases per external adjudication. In the PsA population, none of the patients with confirmed IBD per external adjudication had medical history of IBD. Three patients had events of IBD confirmed by adjudication. One patient had more than 1 event. In the axSpA population, of the 17 patients with adjudicated IBD, 5 patients had a history of IBD.
axSpA, axial spondyloarthritis; IR, incidence rate; IXE, ixekizumab; MACE, major adverse cerebro-cardiovascular events; n, number of patients in each category; PsA, psoriatic arthritis; SAEs, serious adverse events; SMQ, Standardised MedDRA Queries; TEAEs, Treatment-emergent adverse events. Adverse event (AE) terms were derived from MedDRA v23.1. aThe 6 reported deaths in the PsA population were due to cardiovascular event (n=2), metastatic renal cell carcinoma (n=1), cerebrovascular accident (n=1), pneumonia (n=1), and drowning (n=1). In the axSpA population, the causes of deaths were suicide (n=1), sepsis (n=1), and murder (n=1). bOpportunistic infections included esophagus candidiasis, oral candidiasis, hepatitis B reactivation, and herpes zoster. cSome Candida infections cases were also considered as opportunistic infections. All cases of Candida infection were localized. In PsA program, most cases were mild (34/45, IR=1.5 per 100 patient-years) to moderate (10/45, IR=0.4 per 100 patient-years) in severity, except 1 case of esophagus candidiasis considered severe. The patient who reported severe esophagus candidiasis did not discontinue the study drug due to this AE. In axSpA program, all cases were mild (16/26, IR=0.8 per 100 patient-years) or moderate (10/26, IR=0.5 per 100 patient-years) in severity. Across indications, no cases of systemic candidiasis were reported. dNo cases of anaphylaxis were confirmed after medical reviews. eBroad, according to SMQ classification. fBased on neutrophils count lab results. Neutropenia grade ≥3, n (IR) [95%CI] PsA= 8 (0.4) [0.2, 0.7], axSpA= 4 (0.2) [0.1, 0.5]. gThe data represents events confirmed by adjudication. hBroad, according to SMQ or sub-SMQ classification. iThe data represents confirmed cases per external adjudication. In the PsA population, none of the patients with confirmed IBD per external adjudication had medical history of IBD. Three patients had events of IBD confirmed by adjudication. One patient had more than 1 event. In the axSpA population, of the 17 patients with adjudicated IBD, 5 patients had a history of IBD.
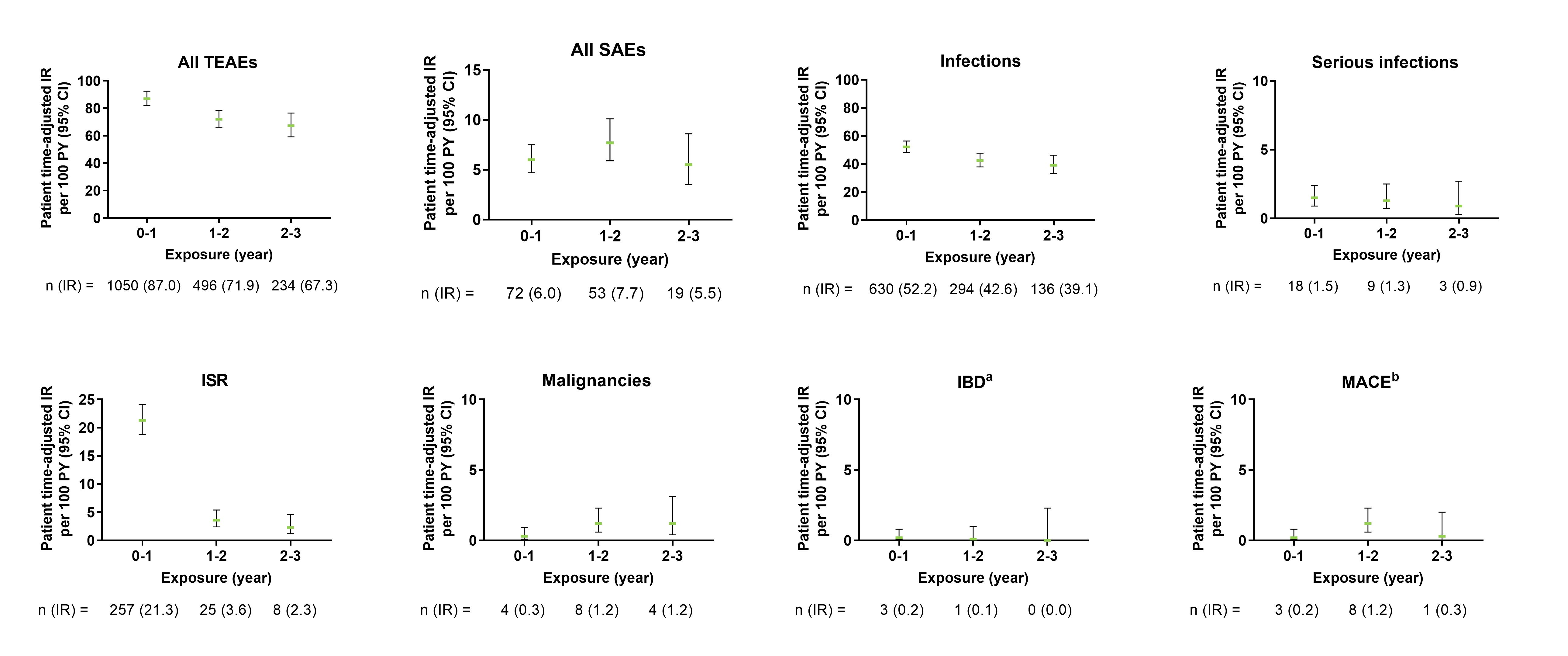 Figure 1. Exposure-adjusted incidence rate of TEAEs, SAEs, and selected AEs in PsA pooled population. The data points on the graph are the IR (95% CI)/100 patient-years at successive year intervals from year 0 to year 3. The CIs for the IRs are from likelihood ratio test of treatment effect from the Poisson regression model. aThe data represents confirmed cases per external adjudication. Three patients had events of IBD confirmed by adjudication. One patient had more than 1 event. bThe data represents events confirmed by adjudication. AEs, adverse events; CI, confidence interval; IBD, inflammatory bowel disease; IR, incidence rates; ISR, injection-site reactions; MACE, major adverse cerebro-cardiovascular events; PsA, psoriatic arthritis; SAE, serious adverse events; TEAEs, treatment-emergent adverse events.
Figure 1. Exposure-adjusted incidence rate of TEAEs, SAEs, and selected AEs in PsA pooled population. The data points on the graph are the IR (95% CI)/100 patient-years at successive year intervals from year 0 to year 3. The CIs for the IRs are from likelihood ratio test of treatment effect from the Poisson regression model. aThe data represents confirmed cases per external adjudication. Three patients had events of IBD confirmed by adjudication. One patient had more than 1 event. bThe data represents events confirmed by adjudication. AEs, adverse events; CI, confidence interval; IBD, inflammatory bowel disease; IR, incidence rates; ISR, injection-site reactions; MACE, major adverse cerebro-cardiovascular events; PsA, psoriatic arthritis; SAE, serious adverse events; TEAEs, treatment-emergent adverse events.
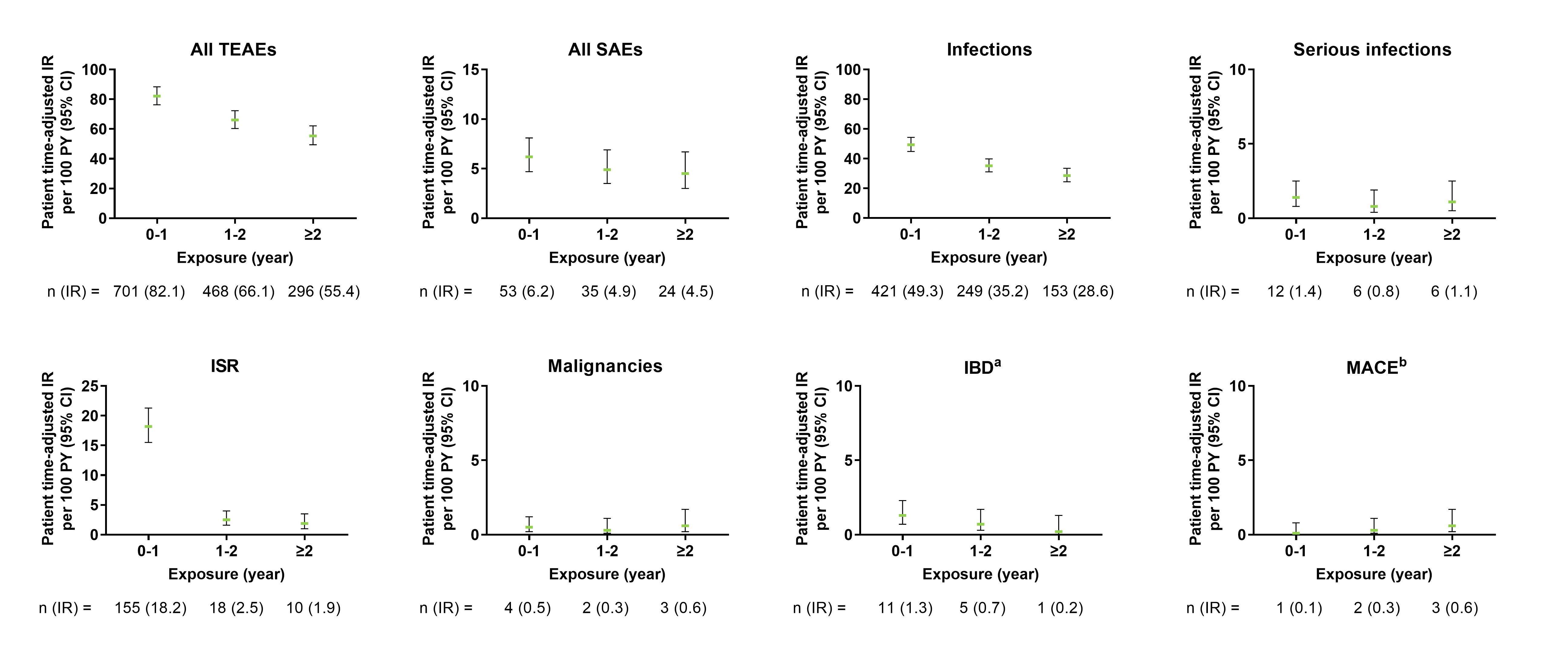 Figure 2. Exposure-adjusted incidence rate of TEAEs, SAEs, and selected AEs in axSpA pooled population. The data points on the graph are the IR (95% CI)/100 patient-years at successive year intervals from year 0 to ≥2 year. The CIs for the IRs are from likelihood ratio test of treatment effect from the Poisson regression model. aThe data represents confirmed cases per external adjudication. bThe data represents events confirmed by adjudication. AEs, adverse events; axSpA, axial spondyloarthritis; CI, confidence interval; IBD, inflammatory bowel disease; IR, incidence rates; ISR, injection-site reactions; MACE, major adverse cerebro-cardiovascular events; SAE, serious adverse events; TEAEs, treatment-emergent adverse events.
Figure 2. Exposure-adjusted incidence rate of TEAEs, SAEs, and selected AEs in axSpA pooled population. The data points on the graph are the IR (95% CI)/100 patient-years at successive year intervals from year 0 to ≥2 year. The CIs for the IRs are from likelihood ratio test of treatment effect from the Poisson regression model. aThe data represents confirmed cases per external adjudication. bThe data represents events confirmed by adjudication. AEs, adverse events; axSpA, axial spondyloarthritis; CI, confidence interval; IBD, inflammatory bowel disease; IR, incidence rates; ISR, injection-site reactions; MACE, major adverse cerebro-cardiovascular events; SAE, serious adverse events; TEAEs, treatment-emergent adverse events.
To cite this abstract in AMA style:
Schwartzman S, Deodhar A, Combe B, Accioly A, Kronbergs A, Janos B, Zhu D, Sandoval Calderon D, Rahman P, Poddubnyy D. Safety Profile of Ixekizumab for the Treatment of Psoriatic Arthritis and Axial Spondyloarthritis up to 3 Years: An Updated Integrated Safety Analysis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-profile-of-ixekizumab-for-the-treatment-of-psoriatic-arthritis-and-axial-spondyloarthritis-up-to-3-years-an-updated-integrated-safety-analysis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-profile-of-ixekizumab-for-the-treatment-of-psoriatic-arthritis-and-axial-spondyloarthritis-up-to-3-years-an-updated-integrated-safety-analysis/
