Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: In patients (pts) with PsA, fatigue is a major driver of perceived impact of disease and has been identified as an important domain to be assessed in clinical trials.1,2 The association between fatigue and other PsA domains (eg, physical function) or clinical response is not well understood. Fatigue was measured with the Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue questionnaire in the pivotal DISCOVER-1 & -2 phase 3 studies of guselkumab (GUS) vs placebo (PBO). This post hoc analysis explores the correlation between FACIT-Fatigue and physical function and clinical response in the DISCOVER program.
Methods: This analysis used pooled data from pts (N=1120) treated with GUS or PBO. In DISCOVER-1 & -2, 381 pts with active PsA (swollen joint count [SJC] ≥3, tender joint count [TJC] ≥3, CRP ≥0.3 mg/dL) and 739 pts with active PsA (SJC ≥5, TJC ≥5, CRP ≥0.6 mg/dL) and inadequate response to standard therapies, respectively, were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at Week (W) 0, W4, then Q8W; or PBO. PBO pts switched to GUS 100 mg Q4W at W24. The FACIT-Fatigue questionnaire has 13 items that assess self-reported fatigue/tiredness over the last 7 days. Items are scored from 0 (very much fatigued) to 4 (not at all fatigued). FACIT-Fatigue response was defined as an increase of ≥4 points from baseline. Physical function was evaluated with the Health Assessment Questionnaire-Disability Index (HAQ-DI). HAQ-DI response was defined as a decrease of ≥0.35 points from baseline. Clinical response was defined as achievement of ACR 20 criteria. Relationships between FACIT-Fatigue and HAQ-DI at W8/16/24 were assessed by Pearson correlation coefficients. Mean changes in HAQ-DI scores at W8/16/24 were summarized in FACIT-Fatigue responders and nonresponders. A logistic regression model was applied to estimate odds ratios (ORs) for achievement of HAQ-DI and ACR 20 response by FACIT-Fatigue response status at each visit. A multiple linear regression model was used to evaluate the association between FACIT-Fatigue and HAQ-DI at W24 after adjusting for SJC, TJC, CRP, and pt assessment of pain.
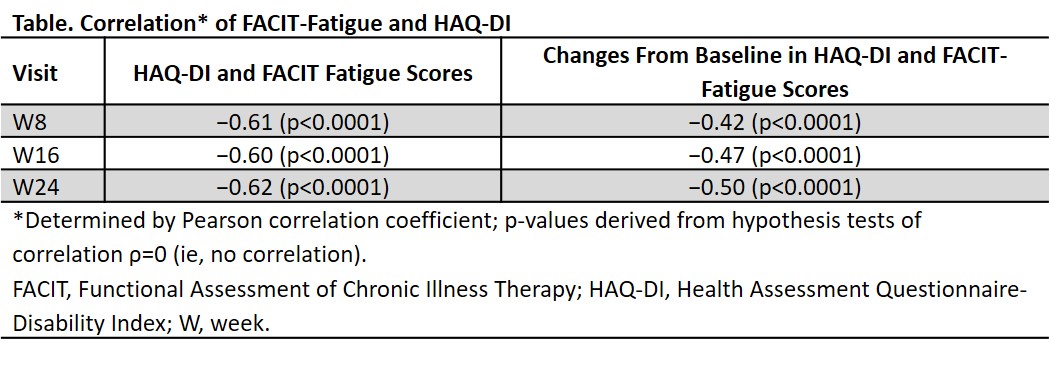
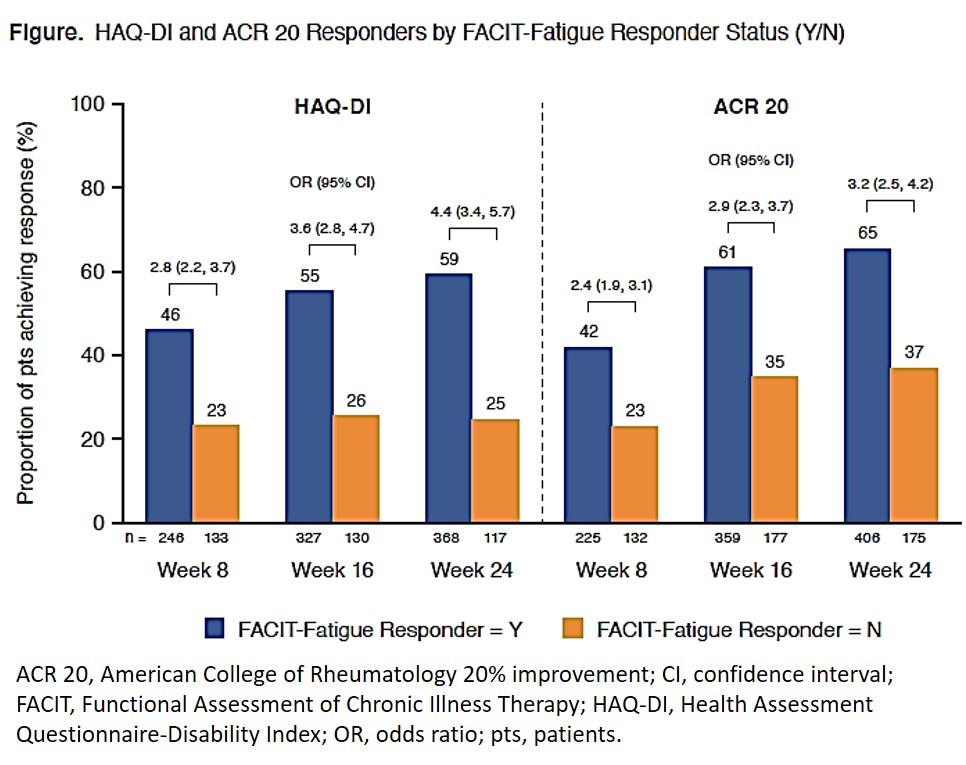
Results: FACIT-Fatigue and HAQ-DI scores and changes from baseline were negatively correlated at W8, W16, and W24 (Table). Mean changes in HAQ-DI were −0.31, −0.43, and −0.48 at W8, W16, and W24, respectively, in FACIT-Fatigue responders and −0.06, −0.07, and −0.09, respectively, in FACIT-Fatigue nonresponders. FACIT-Fatigue responders were significantly more likely than nonresponders to achieve HAQ-DI and ACR 20 response (OR [95% CI] at W8, 2.8 [2.2-3.7] and 2.4 [1.9-3.1]; at W16, 3.6 [2.8-4.7] and 2.9 [2.3-3.7]; and at W24, 4.4 [3.4-5.7] and 3.2 [2.5-4.2], respectively; Figure). Correlations between FACIT-Fatigue and HAQ-DI remained significant after adjusting for SJC, TJC, CRP, and pt assessment of pain.
Conclusion: In pts with PsA, fatigue response is a clinically meaningful predictor of improvements in physical function and achievement of ACR 20 response, reinforcing the importance of assessing fatigue in PsA disease management.
1. Leung YY et al. J Rheumatol. 2020;96:46-9
2. Gudu T et al. Joint Bone Spine. 2016;83:439-43
To cite this abstract in AMA style:
Kavanaugh A, Liu Y, Deodhar A, Rahman P, Mease P, Helliwell P, Gossec L, Kollmeier A, Hsia E, Shawi M, Han C. Correlations Between Reductions in Fatigue Severity and Improvements in Physical Function and Clinical Response in Patients with Psoriatic Arthritis: Results from the Phase 3 DISCOVER Program [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/correlations-between-reductions-in-fatigue-severity-and-improvements-in-physical-function-and-clinical-response-in-patients-with-psoriatic-arthritis-results-from-the-phase-3-discover-program/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/correlations-between-reductions-in-fatigue-severity-and-improvements-in-physical-function-and-clinical-response-in-patients-with-psoriatic-arthritis-results-from-the-phase-3-discover-program/