Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Belimumab is FDA approved for treatment of SLE and Lupus Nephritis. Patients with an eGFR < 30 ml/min/1.73m2 were excluded from clinical trials, thus limiting data on its use in SLE patients with advanced chronic kidney disease (CKD) and end-stage renal disease (ESRD). We describe a case series on the safety and utility of belimumab in these patients.
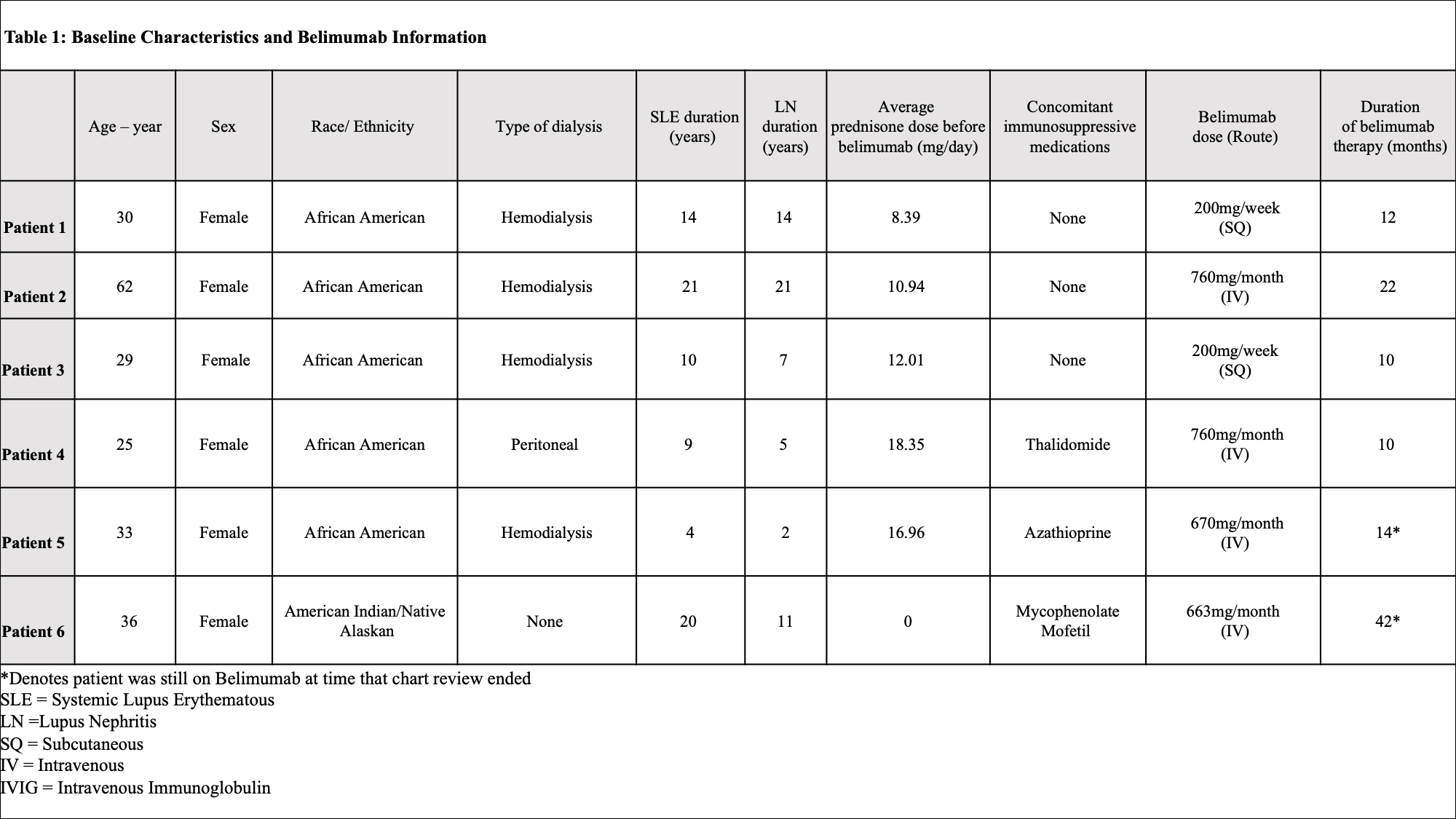
Methods: We conducted a retrospective chart review of patients >18 years old, at Northwell Health, with SLE and CKD stage 4 or worse (sustained eGFR < 30) or ESRD on hemodialysis/peritoneal dialysis. Patients treated with both intravenous and subcutaneous belimumab from 2010 to 2020 were included. We excluded patients with a transient eGFR < 30. Clinical and laboratory data and concomitant immunosuppressive medications were collected in 3-month intervals from 12 months before belimumab initiation to the last time point of belimumab use. The primary endpoint was safety defined by absence of events of interest, including allergic reactions, infections (viral, bacterial, and opportunistic), depression, suicide, and death. As secondary endpoints, we compared median SLEDAI change, serologic parameters, prednisone dose equivalent, and number of flares as determined by SLEDAI SELENA flare index for 12 months before belimumab use and while on belimumab. The only time points that had all data available were analyzed for efficacy.
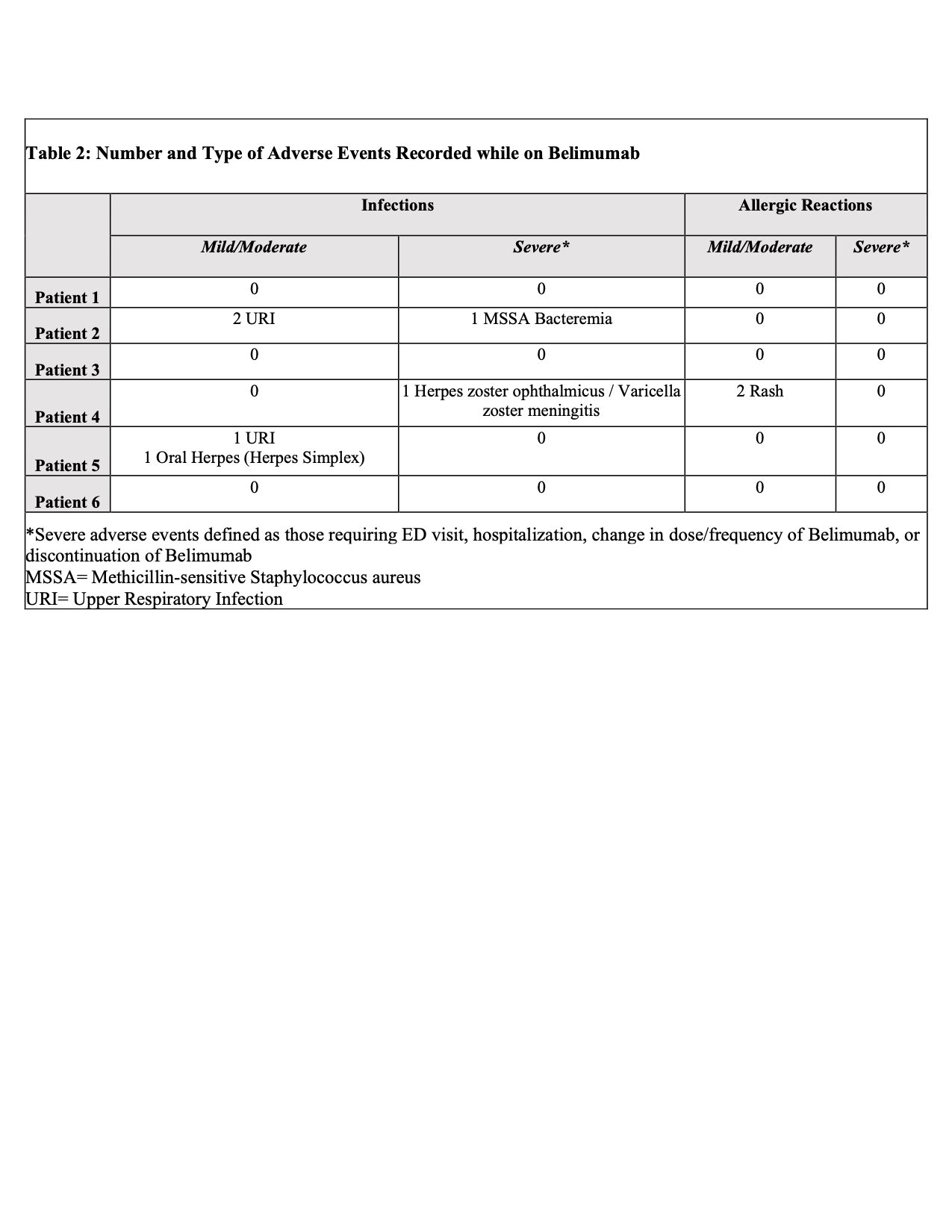
Results: Of 353 patients, we identified six patients with sustained eGFR < 30 or requiring dialysis on belimumab therapy. Baseline demographic and clinical characteristics are recorded in Table 1. The average time of belimumab use in this group was 1.5 years (10-42 months). All patients were alive at the time of analysis. Belimumab was discontinued in 4 patients: 2 for renal transplant, 1 for infectious complications, and 1 for worsening of chronic heart failure. A total of 6 infections were recorded over 9 patient years with multiple events seen in 3 patients. Two infectious events were severe and 4 were mild/moderate. Belimumab was resumed after resolution in all but one patient with systemic H. zoster infection. All patients with infections were on concomitant prednisone (4-10mg/day), 1 patient was on thalidomide (100mg/day), and 1 patient was on azathioprine (150mg/day). No depression, suicide, leukopenia, hypogammaglobulinemia were identified (Table 2). Median SLEDAI score decreased by 50% (from 8 to 4) after 6 months of belimumab use. During the same time frame, median dsDNA titers reduced by more than half (from 390 IU/mL to 167 IU/mL), but there was no change in median complement 4 level (from 18.5mg/dL to 21mg/dL). The cumulative prednisone dose decreased by a mean of 3.75 mg/day (from 13.33 mg/day to 9.58 mg/day) comparing time periods before and while on belimumab (Figure 1). Arthralgia was recorded as improved in two-thirds of patients. There was no change in the median number of flares.
Conclusion: Belimumab is relatively safe in SLE patients with advanced CKD and ESRD on dialysis and may be helpful in reducing SLE activity and steroid use in these patients. However, it is important to be cognizant of the risk of infection, especially with concomitant use of other immunosuppressants.
To cite this abstract in AMA style:
Snyder M, Chen A, Narain S, Marder G. Clinical Use of Belimumab for Systemic Lupus Erythematosus in the Setting of Advanced Chronic Kidney Disease and End-Stage Renal Disease on Dialysis: A Case Series [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/clinical-use-of-belimumab-for-systemic-lupus-erythematosus-in-the-setting-of-advanced-chronic-kidney-disease-and-end-stage-renal-disease-on-dialysis-a-case-series/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-use-of-belimumab-for-systemic-lupus-erythematosus-in-the-setting-of-advanced-chronic-kidney-disease-and-end-stage-renal-disease-on-dialysis-a-case-series/