Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Although giant cell arteritis (GCA) relapse is mostly defined by the recurrence of GCA signs/symptoms, in patients treated only with glucocorticoids, the C-reactive protein (CRP) and the erythrocyte sedimentation rate (ESR) help clinicians assess disease activity. Tocilizumab directly inhibits IL-6-driven acute-phase reactant synthesis in the liver rendering ESR and CRP unreliable for monitoring of disease relapse. Mavrilimumab (MAV), a GM-CSF receptor α inhibitor currently under study for GCA,1 downregulates inflammation upstream of IL-6. Therefore, we hypothesized that MAV would not interfere with the utility of CRP and ESR in monitoring disease activity.
In this study we analyzed the relationship between CRP/ESR and clinical disease activity in GCA patients treated with MAV.
Methods: New-onset and relapsing GCA patients with active disease were recruited. Glucocorticoid-induced remission (no GCA symptoms and CRP < 1 mg/dL or ESR < 20 mm/hr) was required by baseline. Patients were randomized 3:2 to MAV 150 mg or placebo (PBO) subcutaneously every 2 weeks (wks) plus a protocol-defined 26-wk prednisone taper. The primary efficacy endpoint was time to relapse by Wk 26. Relapse (adjudicated) was defined as recurrent GCA signs/symptoms, and/or new/worsening vasculitis on imaging and concurrent CRP ≥1 mg/dL and/or ESR ≥30 mm/hr. CRP and ESR were measured periodically during the trial. This post hoc analysis assessed the CRP and ESR levels from randomization through Wk 26 in patients with and without relapse by treatment arm.
Results: A total of 70 patients were enrolled (MAV, N=42; PBO, N=28). Relapses occurred in 8 (19.1%) and 13 (46.4%) patients in the MAV and PBO groups, respectively. Unequivocal GCA symptoms were present in 20/21 patients with relapse. In the patient without unequivocal GCA symptoms, flare was determined based on worsening vasculitis by imaging. CRP or ESR were elevated at the time of relapse in all cases regardless of treatment arm (Table). Among 34 MAV recipients without relapse, 47.1% had at least one elevated ESR and 29.4% had at least one elevated CRP value. Among 15 PBO recipients without relapse, 66.7% had at least one elevated ESR and 73.3% had at least one elevated CRP value. Overall, at least one elevated inflammatory marker (ESR or CRP) measurement was observed in 58.8% and 93.3% of patients receiving MAV and PBO, respectively (nominal p-values: 0.03 for ESR/CRP; < 0.01 for CRP; 0.32 for ESR).
Conclusion: The observed association of CRP and ESR elevation with recurrent GCA signs/symptoms supports the feasibility of a stringent definition of relapse (i.e., characteristic clinical manifestations plus increased inflammatory markers) in GCA clinical trials. The frequency and magnitude of CRP and ESR elevations at relapse were similar in both treatment groups, indicating that CRP and ESR retain their clinical value under GM-CSF blockade therapy. Transient CRP and ESR elevation without relapse, indicating possible subclinical activity, occurred more often in PBO recipients.
References:
1. Cid, Unizony et al. Arthritis Rheumatol. 2020; 72 (suppl 10)
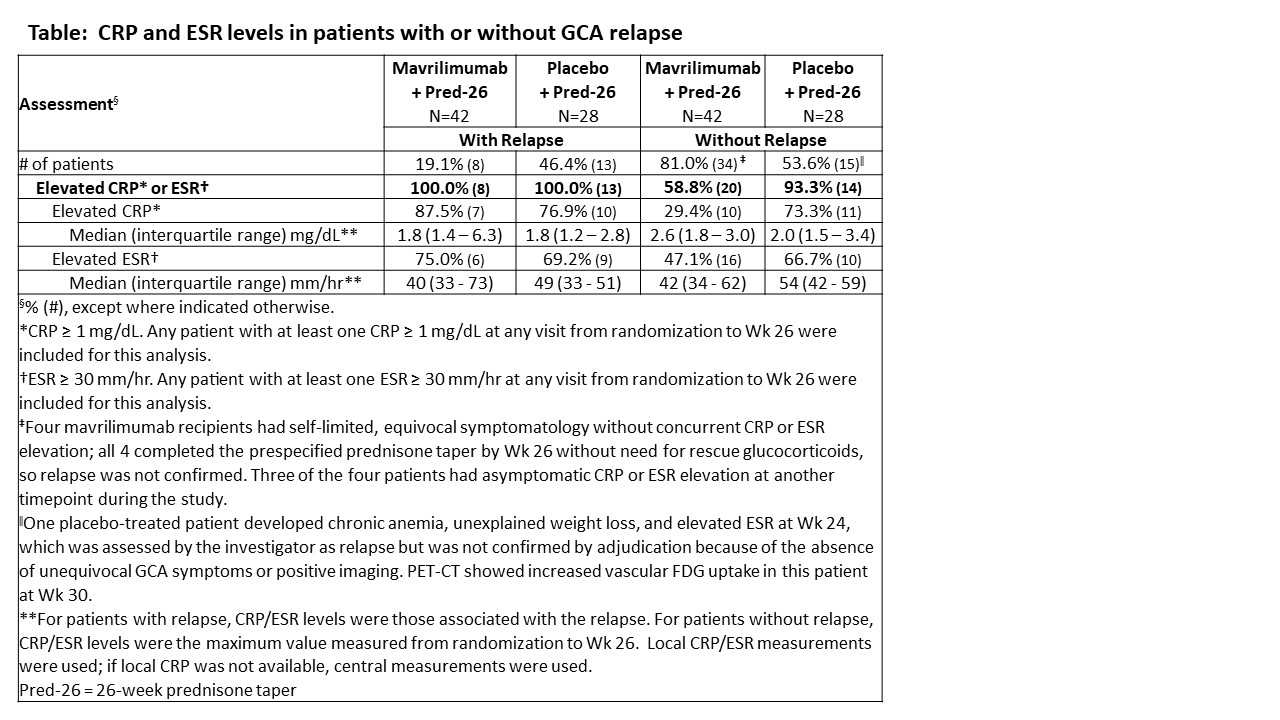 Table: CRP and ESR levels in patients with or without GCA relapse
Table: CRP and ESR levels in patients with or without GCA relapse
To cite this abstract in AMA style:
Unizony S, Cid M, Blockmans D, Brouwer E, Dagna L, Dasgupta B, Hellmich B, Molloy E, Salvarani C, Trapnell B, Warrington K, Wicks I, Samant M, Zhou T, Pupim L, Paolini J. Utility of CRP and ESR in the Assessment of Giant Cell Arteritis Relapse in a Phase 2 Trial of Mavrilimumab [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/utility-of-crp-and-esr-in-the-assessment-of-giant-cell-arteritis-relapse-in-a-phase-2-trial-of-mavrilimumab/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/utility-of-crp-and-esr-in-the-assessment-of-giant-cell-arteritis-relapse-in-a-phase-2-trial-of-mavrilimumab/
