Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Pre-clinical giant cell arteritis (GCA) mouse models have demonstrated effective suppression of arterial wall lesional T-cells through inhibition of Janus kinase 3 (JAK3) and JAK1. However, the use of JAK inhibition in patients with GCA has not been formerly investigated.
Methods: We performed a prospective, open-label, pilot study of baricitinib (4mg/day) in patients with relapsing GCA. The primary outcome was the frequency of adverse events and serious adverse events at week 52. Secondary outcomes included relapse at week 24 and week 52, change in pre-enrollment erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) to week 24 and week 52, and comparison of glucocorticoid dose at enrollment to week 24 and week 52. The study schema is outlined in Figure 1.
Results: 15 patients were enrolled in the study (11, 73% female) with a mean(SD) age at entry 72.4(7.2) years, median (IQR) duration of GCA of 9 (7, 21) months, and median of 1 (1, 2) prior relapse. Treatments prior to study entry included: glucocorticoids (15, 100%); methotrexate (2, 13%); cyclophosphamide (1, 7%); sirukumab (1, 7%). Characteristics at GCA diagnosis and at relapse prior to study entry are listed in Table 1. Four (27%) patients entered the study on prednisone 30mg/day, 6 (40%) at 20 mg/day, and 5 (33%) at 10mg/day. One patient with baseline chronic kidney disease had a decline in renal function below study threshold for continuation and was withdrawn at week 8. The remaining 14 patients completed 52 weeks of baricitinib. At week 52, 14/15 (93%) patients had at least one adverse event recorded with the most frequent events including: infection not requiring antibiotics (n=8), infection requiring antibiotics (n=5), nausea (n=6), leg swelling (n=2), fatigue (n=2), diarrhea (n=1), abdominal pain (n=1). Two patients contracted COVID-19 during the study, both with mild symptoms, neither hospitalized. Only one patient had a serious adverse event during the study (transient thrombocytopenia attributed to concomitant use of antimicrobial therapy).
Study outcomes are highlighted in Table 2. ESR and CRP were significantly lower at week 24 and week 52 compared to pre-enrollment values. Patient global assessment at week 0 was also significantly improved at both week 24 and week 52. Only 1 of 14 (7%) patients relapsed during the study (same patient at week 24 and week 52) The remaining 13 patients achieved steroid discontinuation and remained in disease remission during the duration of the 52-week study. Among patients completing the study, 4/14 (29%) flared during the 12-week follow up period after baricitinib discontinuation.
Conclusion: In this proof of concept study, baricitinib at a dose of 4mg/day appeared both safe and effective in the management of patients with relapsing GCA. Larger randomized clinical trials are needed to determine the utility of JAK inhibition in GCA.
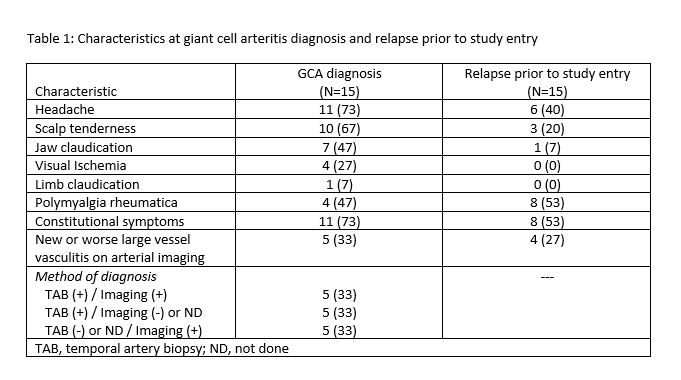 Table 1: Characteristics at giant cell arteritis diagnosis and relapse prior to study entry
Table 1: Characteristics at giant cell arteritis diagnosis and relapse prior to study entry
To cite this abstract in AMA style:
Koster M, Crowson C, Giblon R, Duarte-Garcia A, Jaquith J, Weyand C, Warrington K. Baricitinib in Relapsing Giant Cell Arteritis: A Prospective Open-Label Single-Institution Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/baricitinib-in-relapsing-giant-cell-arteritis-a-prospective-open-label-single-institution-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/baricitinib-in-relapsing-giant-cell-arteritis-a-prospective-open-label-single-institution-study/


