Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: PsA presents with heterogeneous clinical manifestations including joint pain, enthesitis, dactylitis, and skin and nail lesions. Treatment guidelines recommend that PsA patients (pts) who do not adequately respond to conventional synthetic DMARDs (csDMARDs) can initiate treatment with targeted synthetic DMARDs with or without background use of csDMARDs. Deucravacitinib is a novel, oral, selective inhibitor of tyrosine kinase 2 (TYK2) with a unique mechanism of action distinct from other kinase inhibitors. It binds to the TYK2 regulatory domain and allosterically inhibits the enzyme, thereby suppressing signaling of key cytokines (eg, IL-23) involved in the pathogenesis of PsA. In a Phase 2 trial in pts with active PsA, the primary endpoint, ACR 20 response at Week (Wk) 16, was met and the results showed that deucravacitinib was efficacious and well tolerated versus placebo (PBO).1 This analysis further evaluated improvements with deucravacitinib in the Phase 2 trial in PsA pts treated with and without background csDMARDs.
Methods: This 1-year, randomized, double-blind, placebo-controlled, multicenter Phase 2 trial (NCT03881059) enrolled pts who had a PsA diagnosis for ≥6 months, fulfilled Classification Criteria for Psoriatic Arthritis (CASPAR) at screening, had active joint disease (≥3 tender and ≥3 swollen joints), a high-sensitivity CRP level of ≥3 mg/L, and ≥1 plaque psoriasis lesion (≥2 cm). Pts either failed or were intolerant to ≥1 NSAID, corticosteroid, csDMARD, and/or 1 TNF inhibitor (TNFi; up to 30%). Pts were randomized 1:1:1 to deucravacitinib 6 mg once daily (QD) or 12 mg QD, or PBO. A post hoc subgroup analysis in pts with and without background csDMARD use assessed improvements in select clinical outcomes (ACR 20 response, and change from baseline in ACR components, Psoriasis Area and Severity Index total score, and Psoriatic Arthritis Disease Activity Score) at Wk 16.
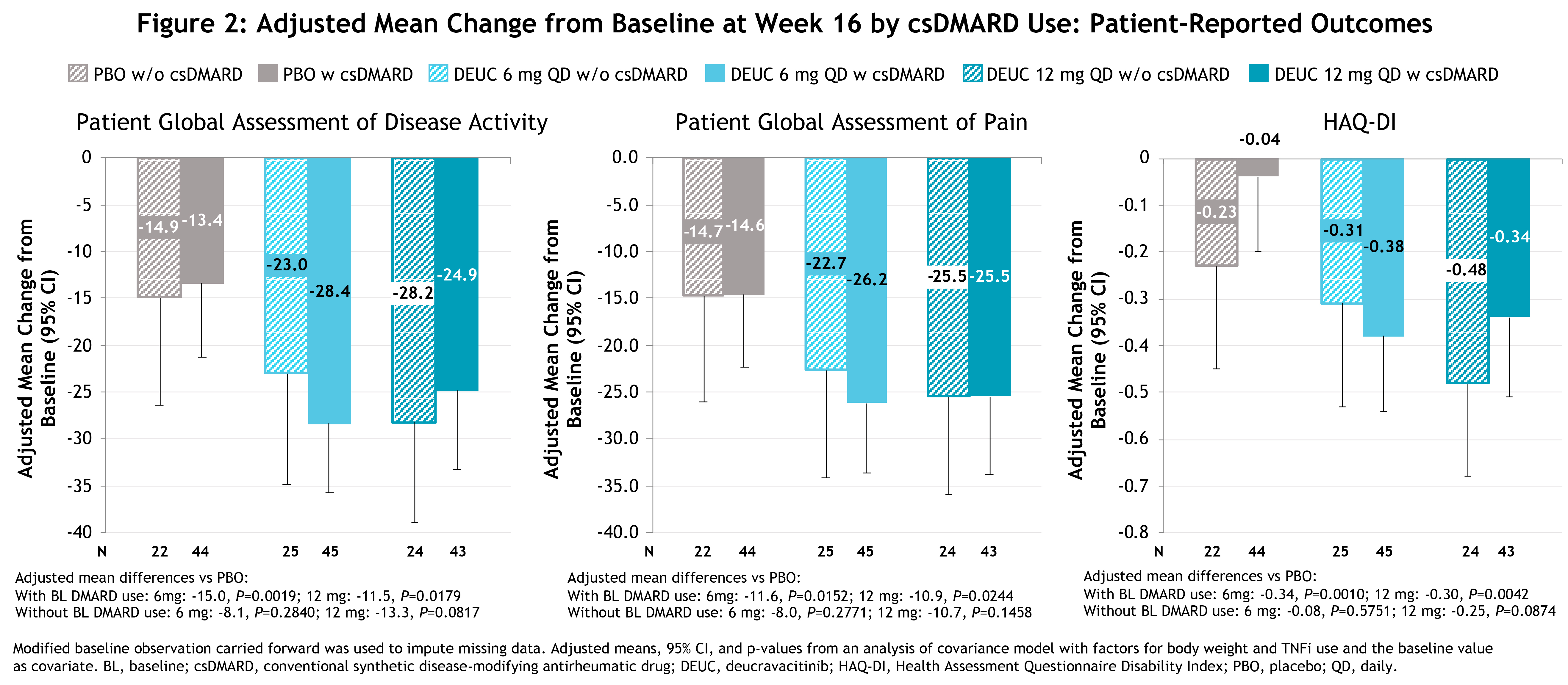
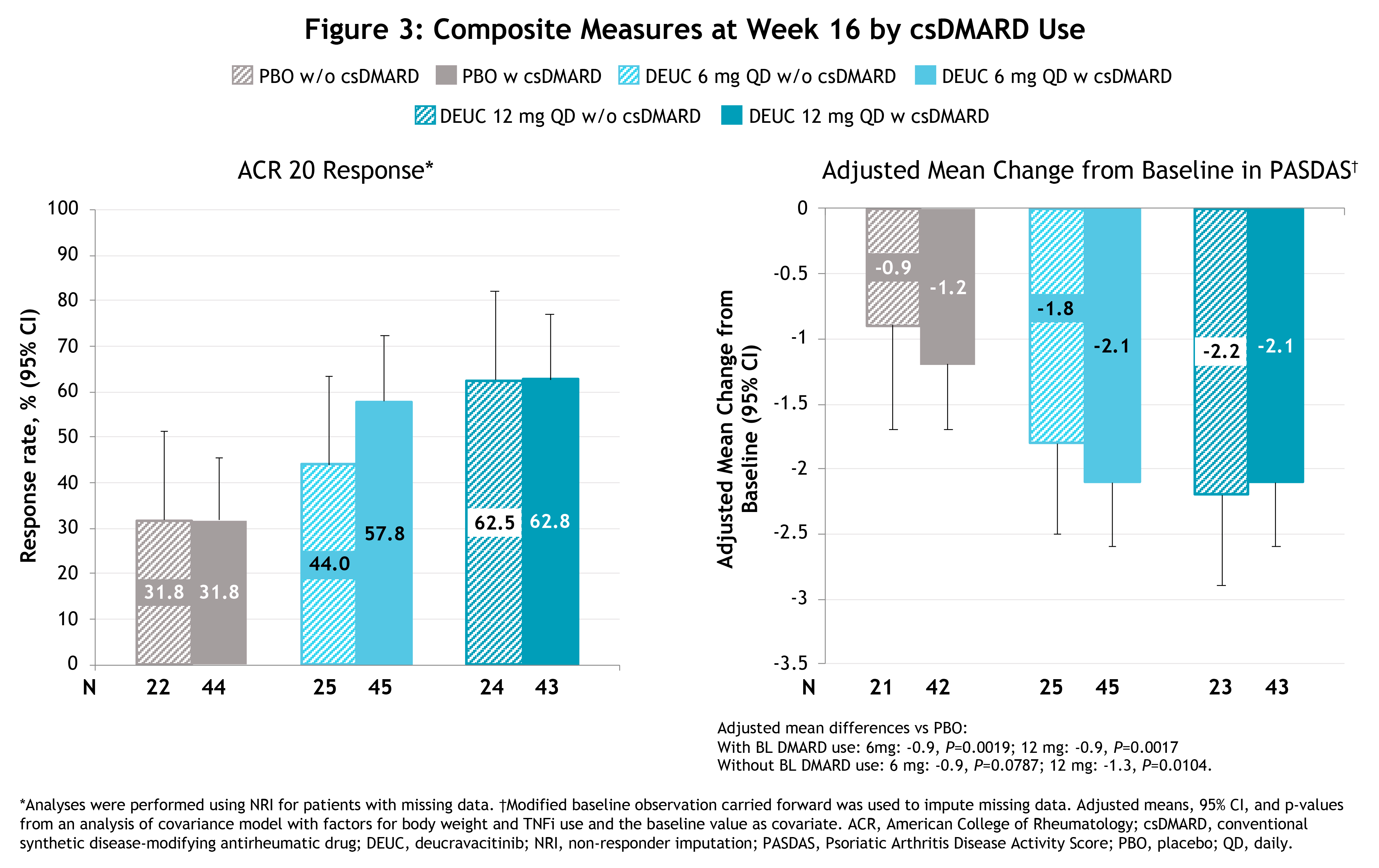
Results: Baseline demographics, clinical characteristics, and disease activity were generally similar among pts with and without background csDMARD use. At baseline, background csDMARD use was 64.3%, 64.2%, and 66.7% in the deucravacitinib 6 mg QD, 12 mg QD, and PBO groups, respectively. Among pts on csDMARDs at baseline, 77.8%, 86%, and 88.6%, respectively, were being treated with methotrexate. Pts with and without background csDMARD use showed similar improvements at Wk 16 with deucravacitinib treatment vs PBO on most clinical measures (Figure 1), pt-reported outcomes (Figure 2), and composite measures (Figure 3). No clinically relevant differences in adverse events (AEs) were observed in pts with or without background csDMARD use.
Conclusion: These analyses demonstrate that the efficacy of deucravacitinib for the treatment of PsA was similar in pts with and without background csDMARD use. The AE profile of deucravacitinib treatment with and without csDMARD use was consistent with findings from the overall Phase 2 PsA trial population.
Reference: 1. Mease PJ et al. Presented at the 2020 ACR Convergence, American College of Rheumatology; Nov 5-9, 2020.
To cite this abstract in AMA style:
Deodhar A, Nowak M, Ye J, Lehman T, Wei L, Banerjee S, Mease P. Deucravacitinib Efficacy in Psoriatic Arthritis (PsA) by Baseline DMARD Use: Exploratory Analysis from a Phase 2 Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/deucravacitinib-efficacy-in-psoriatic-arthritis-psa-by-baseline-dmard-use-exploratory-analysis-from-a-phase-2-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/deucravacitinib-efficacy-in-psoriatic-arthritis-psa-by-baseline-dmard-use-exploratory-analysis-from-a-phase-2-study/