Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Upadacitinib (UPA) 15 mg once daily (QD) has demonstrated efficacy and safety in patients with psoriatic arthritis (PsA) for up to 56 weeks in the Phase 3 SELECT-PsA 1 and 2 trials.1,2 This post hoc analysis of these studies explored the association of baseline characteristics and short-term responses with achievement of minimal disease activity (MDA) and Disease Activity Index for Psoriatic Arthritis (DAPSA) low disease activity (LDA).
Methods: Data were pooled from patients with prior inadequate response or intolerance to ≥1 non-biologic (b) DMARDs (SELECT-PsA 1) or ≥1 bDMARDs (SELECT-PsA 2) originally randomized to UPA 15 mg QD. Logistic regression models were used to assess the association between baseline characteristics and short-term (Week 12) responses with achieving MDA or DAPSA LDA at 56 weeks, sustained MDA (MDA at Weeks 36 and 56), or sustained DAPSA LDA (DAPSA LDA at Weeks 36, 44, and 56). Each predictor was evaluated separately in an initial model that included effects for study and concurrent non-bDMARD use. Odds ratios and concordance (c-)statistics were used to determine the predictive accuracy. Statistically significant predictors were then evaluated simultaneously using stepwise logistic regression with the Akaike Information Criterion for model-building.
Results: Of 640 patients included in the analysis, 40% and 47% achieved MDA and DAPSA LDA, respectively, at 56 weeks. Evaluated separately, younger age, sex (male), geographic region, lower weight, lower body mass index, the presence of dactylitis or enthesitis, and lower scores of Patient’s Assessment of Pain (Pt-Pain), Patient’s Global Assessment (PtGA), tender joint count in 68 joints, and Health Assessment Questionnaire-Disability Index (HAQ-DI) were significant baseline predictors for achieving MDA and DAPSA LDA at Week 56. Lower Pt-Pain (Weeks 12–24) and PtGA (Weeks 16–24) scores were strongly predictive (c-statistics >0.8) of achieving MDA at Week 56, and both measures (from Week 8) were moderately predictive (c-statistics >0.7) of achieving DAPSA LDA. Evaluated simultaneously with several baseline characteristics, lower Pt-Pain and HAQ-DI scores at Week 12 were included in models strongly predictive of achieving MDA (c-statistic=0.850; Figure 1) and DAPSA LDA (c-statistic=0.840; Figure 2) at Week 56. For each 1-point increase in Pt-Pain or HAQ-DI scores at Week 12 (after adjusting for other effects in the model), patients were less likely to achieve MDA (by 32% or 56%, respectively) or DAPSA LDA (by 23% or 31%, respectively) at Week 56. Predictors for achieving sustained MDA and sustained DAPSA LDA were generally similar to those identified for achieving MDA and DAPSA LDA, respectively.
Conclusion: In patients with PsA receiving UPA 15 mg, baseline characteristics and early responses strongly predicted achievement of MDA or DAPSA LDA at Week 56. This may guide considerations of treatment targets in clinical trials and encourage physicians to further optimize treatment of their patients in clinical practice.
1. McInnes IB, et al. Ann Rheum Dis 2020;79:16–7.
2. Mease PJ, et al. Rheumatol Ther 2021 [Epub ahead of print].
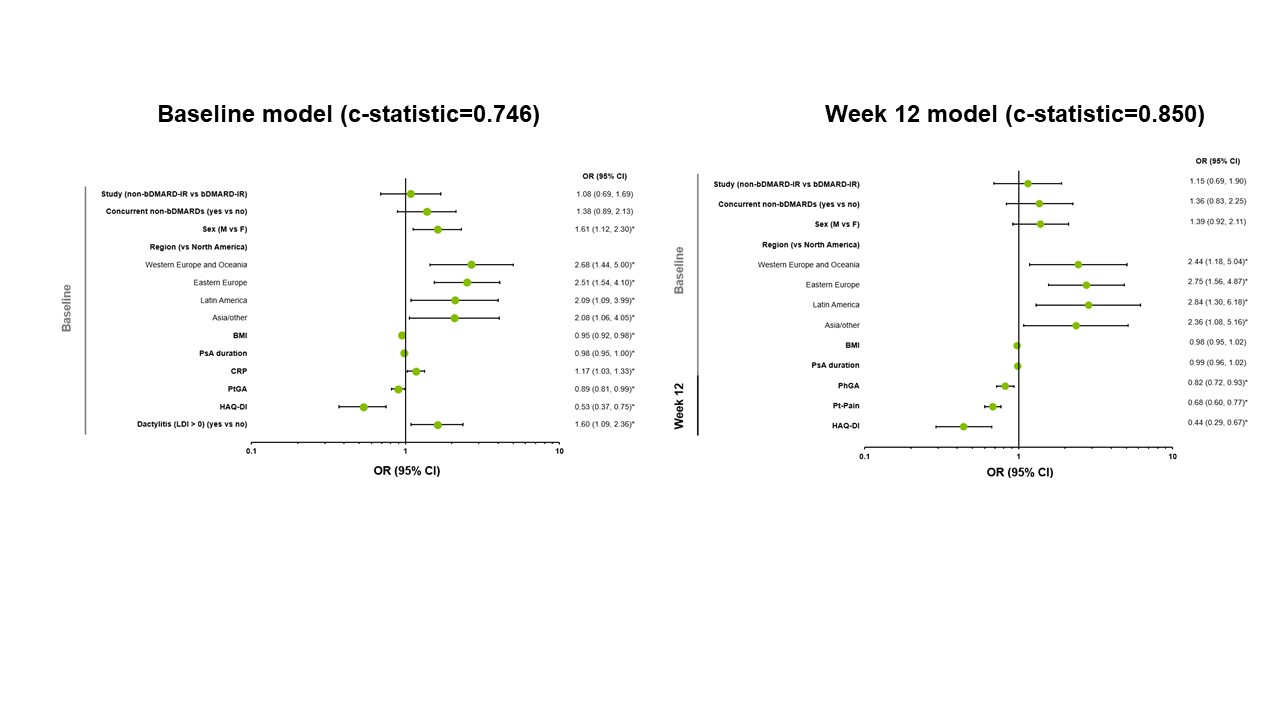 Figure 1. Predictors of Achievement of MDA at Week 56 with Upadacitinib 15 mg – Evaluated Simultaneously. *p < 0.05. c-statistics: 0.6 indicates a fair model, 0.7 indicates a good model, and 0.8 indicates a strong model. Non-responder imputation was used for MDA at Week 56. bDMARD, biologic disease-modifying antirheumatic drug; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; F, female; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate responder; LDI, Leeds Dactylitis Index; M, male; MDA, minimal disease activity; OR, odds ratio; PhGA, Physician’s Global Assessment; PsA, psoriatic arthritis; PtGA, Patient’s Global Assessment; Pt-Pain, Patient’s Assessment of Pain.
Figure 1. Predictors of Achievement of MDA at Week 56 with Upadacitinib 15 mg – Evaluated Simultaneously. *p < 0.05. c-statistics: 0.6 indicates a fair model, 0.7 indicates a good model, and 0.8 indicates a strong model. Non-responder imputation was used for MDA at Week 56. bDMARD, biologic disease-modifying antirheumatic drug; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; F, female; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate responder; LDI, Leeds Dactylitis Index; M, male; MDA, minimal disease activity; OR, odds ratio; PhGA, Physician’s Global Assessment; PsA, psoriatic arthritis; PtGA, Patient’s Global Assessment; Pt-Pain, Patient’s Assessment of Pain.
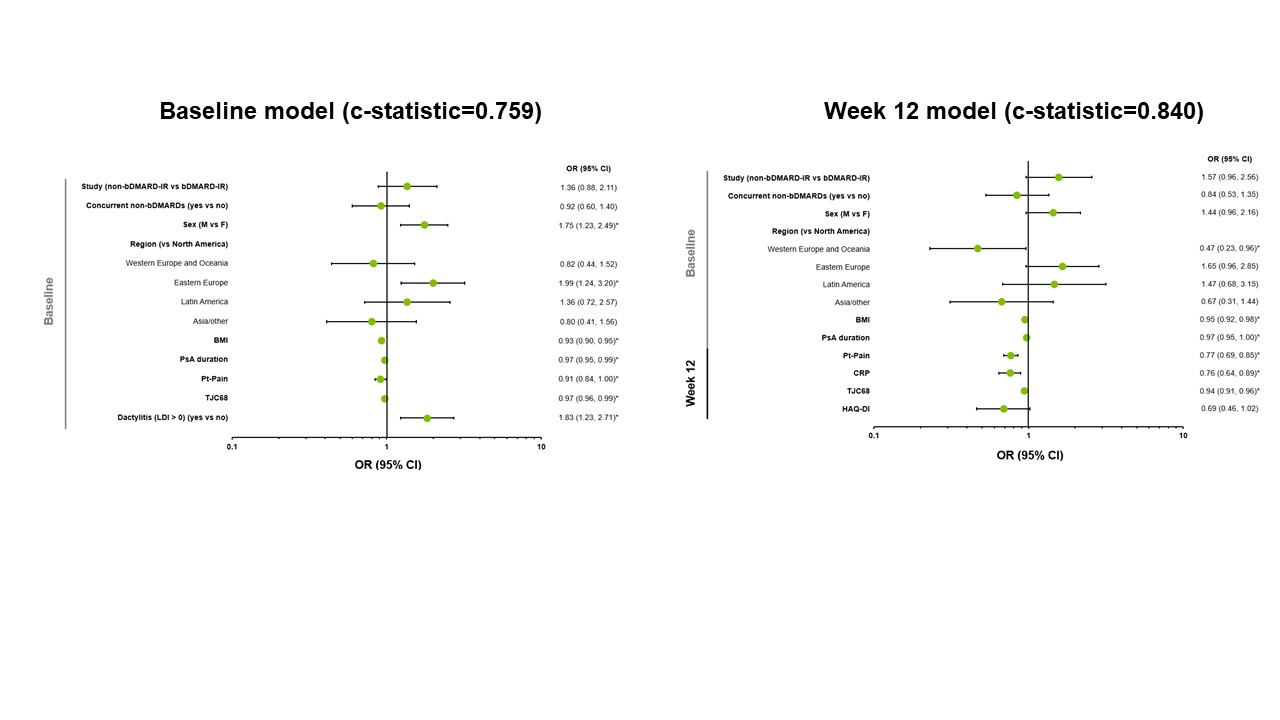 Figure 2. Predictors of Achievement of DAPSA LDA at Week 56 with Upadacitinib 15 mg – Evaluated Simultaneously. *p < 0.05. c-statistics: 0.6 indicates a fair model, 0.7 indicates a good model, and 0.8 indicates a strong model. Non-responder imputation was used for DAPSA LDA at Week 56. bDMARD, biologic disease-modifying antirheumatic drug; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; DAPSA, Disease Activity Index for Psoriatic Arthritis; F, female; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate responder; LDA, low disease activity; LDI, Leeds Dactylitis Index; M, male; OR, odds ratio; PsA, psoriatic arthritis; Pt-Pain, Patient’s Assessment of Pain; TJC68, tender joint count in 68 joints.
Figure 2. Predictors of Achievement of DAPSA LDA at Week 56 with Upadacitinib 15 mg – Evaluated Simultaneously. *p < 0.05. c-statistics: 0.6 indicates a fair model, 0.7 indicates a good model, and 0.8 indicates a strong model. Non-responder imputation was used for DAPSA LDA at Week 56. bDMARD, biologic disease-modifying antirheumatic drug; BMI, body mass index; CI, confidence interval; CRP, C-reactive protein; DAPSA, Disease Activity Index for Psoriatic Arthritis; F, female; HAQ-DI, Health Assessment Questionnaire-Disability Index; IR, inadequate responder; LDA, low disease activity; LDI, Leeds Dactylitis Index; M, male; OR, odds ratio; PsA, psoriatic arthritis; Pt-Pain, Patient’s Assessment of Pain; TJC68, tender joint count in 68 joints.
To cite this abstract in AMA style:
Aletaha D, Mease P, Lippe R, Behrens F, Haaland D, Palominos P, Lertratanakul A, Lane M, Douglas K, Nash P, Kavanaugh A. Predictors for Achievement of Low Disease Activity at Week 56 in Patients with Psoriatic Arthritis Who Received Upadacitinib 15 Mg Once Daily: Pooled Analysis of Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/predictors-for-achievement-of-low-disease-activity-at-week-56-in-patients-with-psoriatic-arthritis-who-received-upadacitinib-15-mg-once-daily-pooled-analysis-of-two-phase-3-studies/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-for-achievement-of-low-disease-activity-at-week-56-in-patients-with-psoriatic-arthritis-who-received-upadacitinib-15-mg-once-daily-pooled-analysis-of-two-phase-3-studies/
