Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: In the SELECT-PsA 1 study, through 24 weeks (wks), once daily upadacitinib 15 mg (UPA15) and 30 mg (UPA30) showed improvements in musculoskeletal symptoms, psoriasis, physical function, pain, fatigue, and quality of life, as well as inhibition of radiographic progression in patients (pts) with psoriatic arthritis (PsA) and inadequate response or intolerance to ≥1 non-biologic disease-modifying antirheumatic drug (DMARD).1 This analysis reports the efficacy and safety of UPA vs adalimumab (ADA) up to 56 wks from the ongoing long-term extension of SELECT-PsA 1.
Methods: Pts received UPA15 or UPA30, ADA 40mg every other wk for 56 wks, or PBO through wk 24 switched thereafter to either UPA15 or UPA30 until wk 56. Efficacy endpoints as listed and defined in the Table were analyzed at wk 56. Results for binary endpoints are based on non-responder imputation analysis; treatments were compared using the Cochran-Mantel-Haenszel test. Results for non-radiographic continuous endpoints are based on mixed model repeated measures model based on as observed data. Radiographic endpoints were analyzed based on linear extrapolation. Treatment-emergent adverse events (TEAEs) per 100 pt years (PY) were summarized for pts who received ≥1 dose of study drug.
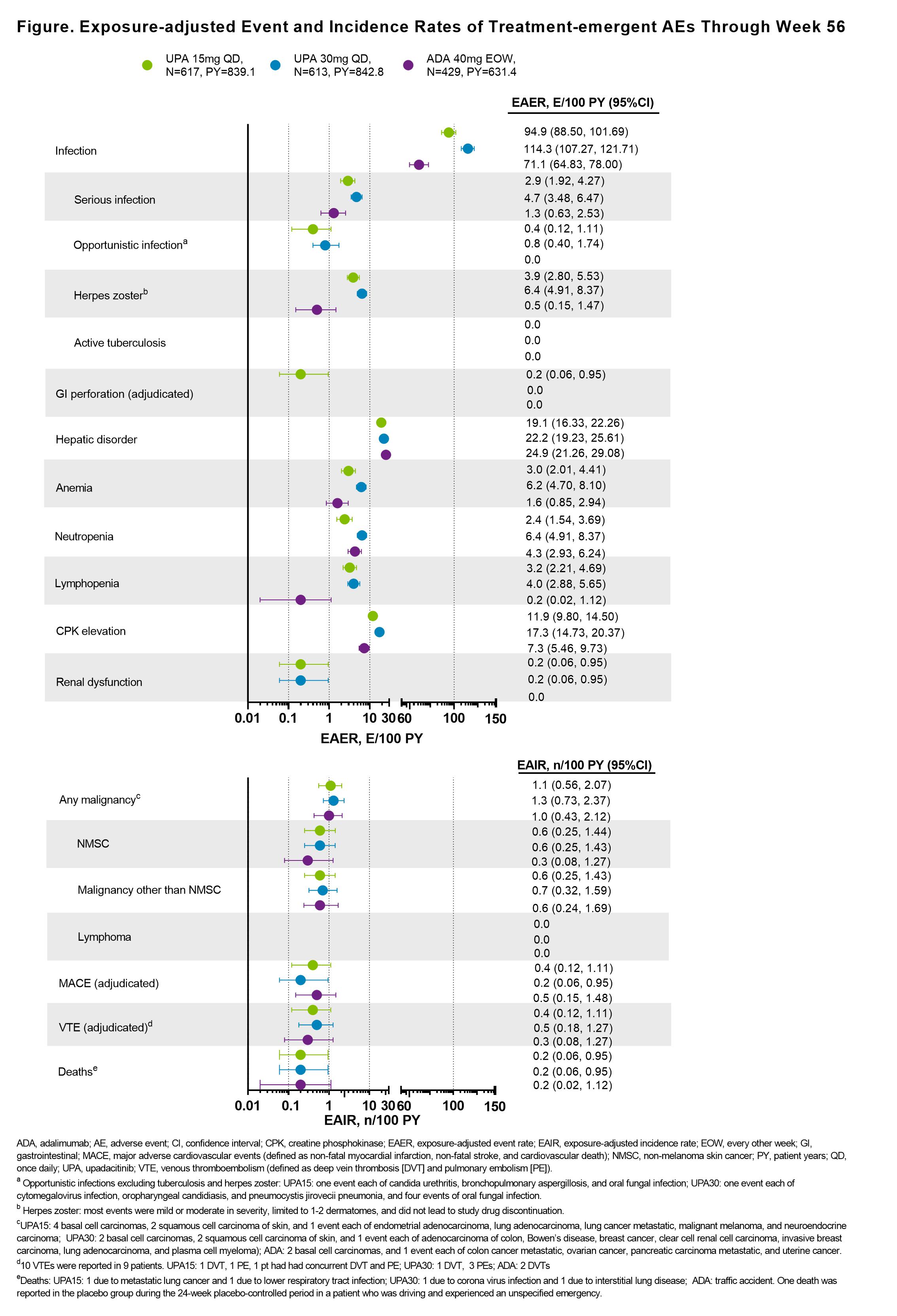
Results: Of 1704 pts who received ≥1 dose of study drug, 1419 (83.2%) completed 56 wks of treatment on study drug. Across all treatment groups, the proportions of pts who had achieved ACR20/50/70, MDA, PASI75/90/100, resolution of enthesitis, and resolution of dactylitis were maintained or further improved from wk 241 through wk 56; these proportions were generally greater for pts originally randomized to UPA vs ADA (Table). At wk 56, mean change from BL in mTSS was similar with UPA15, UPA30, and ADA. Improvements in pts who switched from PBO to UPA were generally similar to those originally randomized to UPA at wk 56. Through wk 56, the rates of TEAEs and serious AEs, including serious infections, were similar in the UPA15 and ADA arms and higher with UPA30 (Figure). The rate of herpes zoster was higher with UPA vs ADA in a dose-dependent manner. Malignancies were reported at similar rates among all treatment groups. Adjudicated venous thromboembolic events and major adverse cardiovascular events were reported in all groups with comparable rates. Two deaths were reported with UPA15, 2 with UPA30, and 1 with ADA; 1 death was reported with PBO during the 24-wk PBO-controlled period.
Conclusion: Efficacy responses were maintained or further improved with UPA15 and UPA30 over 56 wks and were numerically higher for pts originally randomized to UPA vs ADA. The inhibition of radiographic progression was maintained at wk 56 and was similar with UPA and ADA. At wk 56, improvements in efficacy were observed in pts who switched from PBO to UPA. No new safety findings were observed with longer exposure to UPA.
References:
1. McInnes IB et al. Ann Rheum Dis, 2020; 79:12.
To cite this abstract in AMA style:
McInnes I, Kato K, Magrey M, Merola J, Kishimoto M, Pacheco Tena C, Haaland D, Chen L, Duan Y, Zueger P, Liu J, Lippe R, Pangan A, Behrens F. Long-Term Safety and Effectiveness of Upadacitinib in Patients with Psoriatic Arthritis: Results at 56 Weeks from the SELECT-PsA 1 Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-effectiveness-of-upadacitinib-in-patients-with-psoriatic-arthritis-results-at-56-weeks-from-the-select-psa-1-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-effectiveness-of-upadacitinib-in-patients-with-psoriatic-arthritis-results-at-56-weeks-from-the-select-psa-1-study/