Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Fibromyalgia & Other Clinical Pain Syndromes (0474–0477)
Session Type: Abstract Session
Session Time: 11:45AM-12:00PM
Background/Purpose: Fibromyalgia (FM) is characterized by chronic widespread pain, fatigue, and nonrestorative sleep, a symptom constellation suggestive of a pathological disturbance in central pain processing known as central sensitization. TNX-102 SL* (cyclobenzaprine HCl sublingual tablets, ‘TNX’) targets improvement in sleep quality in order to reverse central sensitization. Prior studies of TNX at a 2.8 mg dose in FM showed signals for broad efficacy but narrowly missed significance on primary outcome of daily diary pain reduction. This Phase 3 trial (‘RELIEF’#) evaluated efficacy and safety of TNX for FM at twice the dose, 5.6 mg.
Methods: The Intent-to-treat sample of 503 patients meeting 2016 FM diagnostic criteria were enrolled at 39 U.S. sites and received TNX 2.8 mg or placebo for 2 weeks followed by TNX 5.6 mg or placebo for 12 weeks. Primary outcome measure was change from baseline in weekly average of daily diary pain scores (0-10 NRS) at Week 14, analyzed by mixed model repeated measures with multiple imputation (MMRM-MI). The 1st key secondary analysis was Patient Global Impression of Change (PGIC) responders by logistic regression. Remaining key secondaries (by MMRM-MI) included: Fibromyalgia Impact Questionnaire-Revised (FIQ-R) symptom domain; FIQ-R function domain; Patient Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance; PROMIS Fatigue; and daily diary NRS of sleep quality. To adjust for multiplicity, a sequential, ordered test procedure was applied to each (at p< 0.045 to account for an interim analysis α-spend).
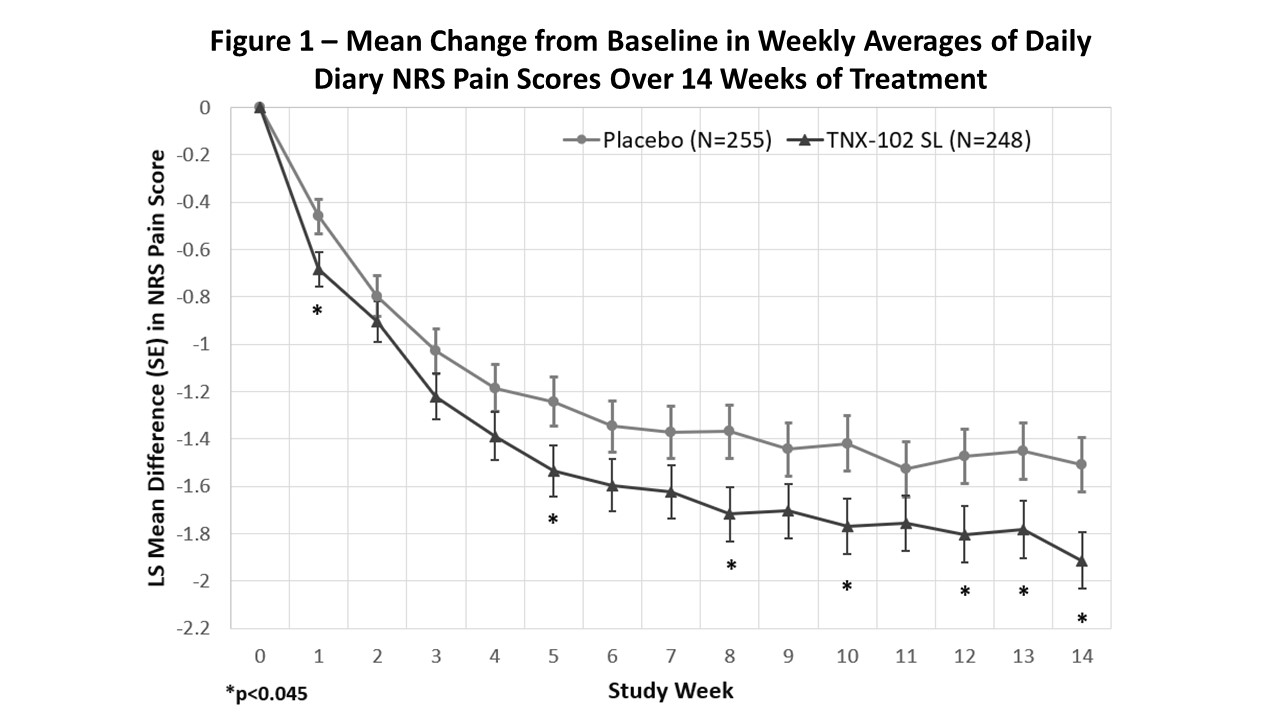
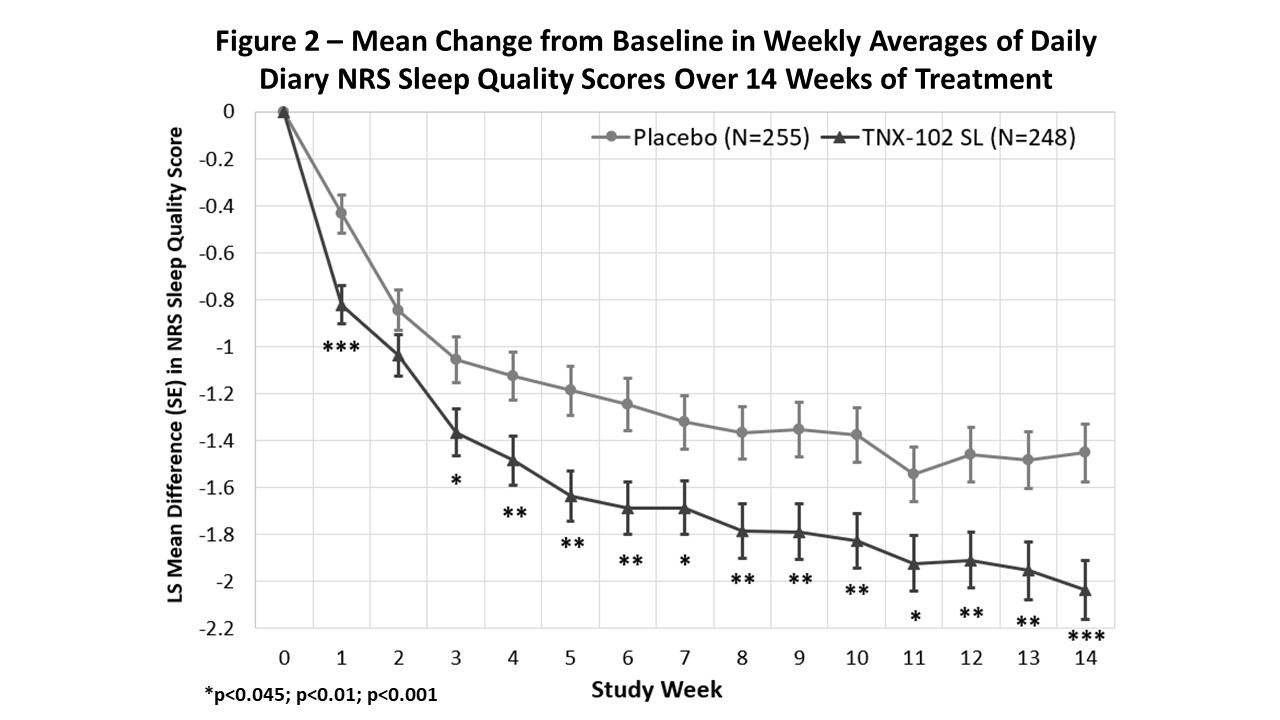
Results: TNX was effective in decreasing daily diary pain compared to placebo (p=0.010; Fig. 1). A ≥30% pain reduction responder analysis indicated 46.8% TNX responders vs. 34.9% on placebo (p=0.006). By PGIC, 37.5% on TNX were responders, which was numerically but not significantly greater than 29.4% on placebo (p=0.058). TNX also provided greater separation from placebo on: FIQ-R Symptoms (p=0.007), Function (p=0.009); PROMIS Sleep Disturbance (p< 0.001) and Fatigue (p=0.018); and daily diary sleep quality (p< 0.001; Fig. 2).
In the TNX group, 82.3% completed vs. 83.5% on placebo. Systemic adverse events (AEs) were infrequent, with somnolence/sedation the only category at a rate of ≥ 5% on TNX (5.6% TNX; 1.2% placebo). The most common local administration site reaction was oral numbness (17.3% TNX; 0.8% placebo) which was episodic and typically temporally related to dosing, resolving in < 60 min in most occurrences. AEs led to premature study discontinuation in 8.9% on TNX vs. 3.9% on placebo.
Conclusion: Bedtime TNX at the 5.6 mg dose significantly reduced daily pain, provided a larger rate of ≥30% pain responders. It showed activity in improving sleep, fatigue, and other FM symptoms and measures of function. In addition, nightly TNX 5.6 mg was well tolerated. Taken together, these findings indicate TNX-102 SL primarily targets sleep quality and may thereby reduce central sensitization, leading to improvement at the syndromal level, manifesting as broad-spectrum activity across symptoms of FM.
To cite this abstract in AMA style:
Sullivan G, Kelley M, Iserson A, Peters P, Peters A, Flint C, Gendreau J, Harris H, Vaughn B, Lederman S. TNX-102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia in the RELIEF Study: Positive Results of a Phase 3 Randomized, Double-Blind, Placebo-Controlled Multicenter Efficacy and Safety Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/tnx-102-sl-sublingual-cyclobenzaprine-for-the-treatment-of-fibromyalgia-in-the-relief-study-positive-results-of-a-phase-3-randomized-double-blind-placebo-controlled-multicenter-efficacy-and-safet/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tnx-102-sl-sublingual-cyclobenzaprine-for-the-treatment-of-fibromyalgia-in-the-relief-study-positive-results-of-a-phase-3-randomized-double-blind-placebo-controlled-multicenter-efficacy-and-safet/