Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Familial Mediterranean Fever (FMF) is the most common auto-inflammatory disease characterized by chronic inflammation, recurrent episodes of fever, abdominal and thoracic pain due to serositis, skin irritations and arthralgia. FMF patients are at risk for severe organ failure due to amyloidosis. Colchicine and IL-1 antagonists are approved therapeutic options but alternative treatment options are still warrant. Tocilizumab (TCZ) is a monoclonal antibody directed against the IL-6 receptor and approved for several other autoimmune diseases that go along with high inflammation markers.
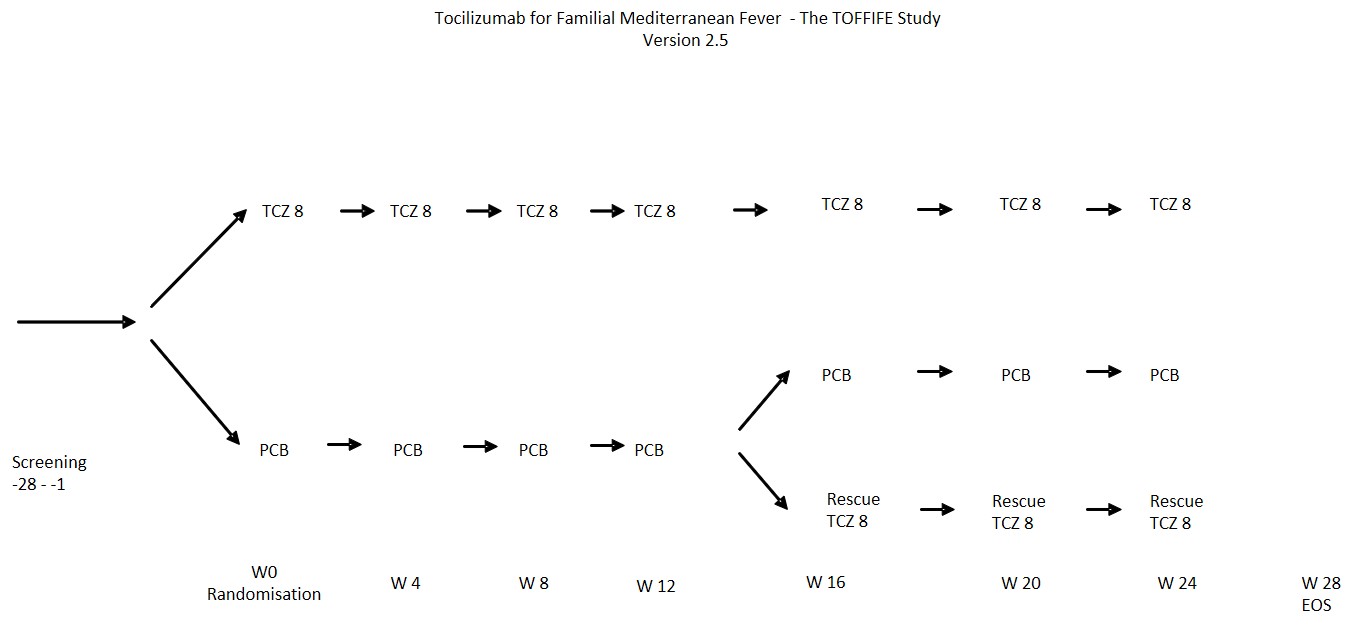
Methods: This was a randomized, blinded, placebo-controlled trial conducted at 5 German centres. Adult patients with FMF, who had at least one known mutation of the MEFV gene and active disease with inadequate response or intolerance to colchicine were included. The physician’s global assessment of disease activity (PGA) based on a 5 point-scale was used as a clinical score and had to be >2 at screening. Patients were randomized 1:1 to either receive TCZ intravenously with 8 mg/kg bodyweight or placebo over a period of 24 weeks. Patients with inadequate response after week 12 had the opportunity to receive open label TCZ at week 16. The primary endpoint was the number of patients achieving an adequate response to treatment at week 16, defined as a PGA ≤ 2 + normalized ESR or CRP + normalized SAA. Patients filled out a patient’s diary to help the investigators to judge the PGA
Results: Of the 31 included patients, only 25 were randomized, 6 patients dropped out before randomization due to missing data, withdrawal or incompliance. For further evaluation the intention to treat population consisted of 25 patients with a median age of 31 years (range 18 – 53y), of which 14 (56%) were female. In their medical history all were treated with colchicine with inadequate response, whereas in 3 patients anakinra and in 1 patient canakinumab were ineffective. At week 16, the primary endpoint was met by only 2 patients in the TCZ, but none of the patients in the placebo arm (P-value of 0.089). The difference was primarily due to the failed normalization of the laboratory parameters in the placebo group, whereas the difference in PGA was not significant. No new safety aspects occurred.
Conclusion: This is the first randomized, placebo-controlled study on the efficacy of TCZ in patients with active FMF. Our results demonstrate the superiority with regard to our primary endpoint: more patients in the TCZ arm experienced an adequate response to treatment in comparison to placebo. SAA levels only normalized in TCZ treated patients. As TCZ normalizes CRP levels in almost all patients this effectiveness has to be confirmed in a larger trial.
To cite this abstract in AMA style:
Henes J, Saur S, Kofler D, Krusche M, Xenitidis T, Meisner C, Kedor C, Koetter I, Schulze-Koops H, Feist E. Tocilizumab for the Treatment of Familial Mediterranean Fever – a Randomized, Double Blind, Placebo-controlled Phase II Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/tocilizumab-for-the-treatment-of-familial-mediterranean-fever-a-randomized-double-blind-placebo-controlled-phase-ii-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tocilizumab-for-the-treatment-of-familial-mediterranean-fever-a-randomized-double-blind-placebo-controlled-phase-ii-study/