Session Information
Date: Monday, November 9, 2020
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Tocilizumab (TCZ) suppresses CRP. Thus, CRP cannot be used as a marker for disease activity in GCA patients treated with TCZ and monitoring of disease activity is based on clinical signs and symptoms only. Novel methods to monitor disease activity in GCA under IL-6 blocking therapies are needed.
The Giant Cell Arteritis treated with Ultra-Short Glucocorticoids and TOcilizumab (GUSTO) study (NCT03745586) offered a unique opportunity to test monitoring of the intima-media thickness (IMT) of arterial vessel walls with ultrasound (US) as a surrogate for disease activity.
Methods: In this single-arm, single-center, open-label clinical trial, 18 patients with newly diagnosed GCA were enrolled. They received 500 mg methylprednisolone intravenously for three consecutive days. Thereafter, glucocorticoids were discontinued and TCZ (8mg/kg body weight) was administered intravenously, followed by weekly subcutaneous TCZ injections (162 mg) from day 10 to week 52. Change of IMT was an exploratory secondary outcome.
US of temporal (TA), axillary (AA) and subclavian (SA) arteries was performed at baseline, on day 2-3, at week 4, 8, 12 and 24. The sonographer was not blinded. The maximum IMT of the compressed TA (mainstem, frontal/parietal branch) and single wall IMT at landmarks of AA/SA were measured with an 18-22 or 9-11 MHz probe in B-mode. Biopsied branches and incompressible TA mainstems were excluded from follow-up. Diagnostic cut-offs and mean normal values for IMT of TA and AA were taken from Schäfer et al. (Rheumatology 2017;56(9):1479‐1483). For the SA, the diagnostic cut-off and mean normal IMT of the AA were used. For each examination, the IMT of each TA segment was standardized by dividing it by (2 x (mean normal IMT)), the sum of these values was then divided by the number of segments. The same was done for the AA/SA separately.
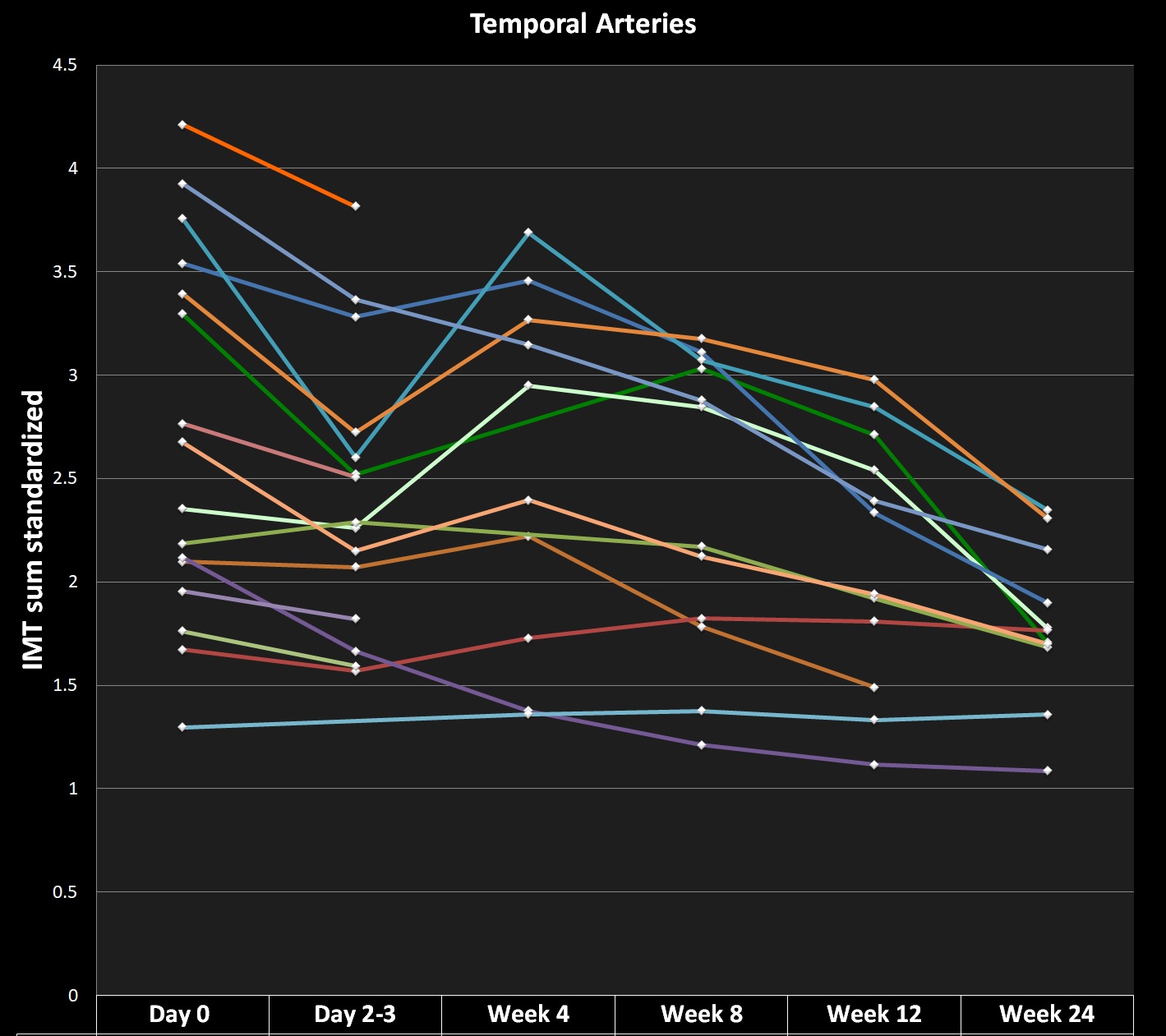
Results: According to diagnostic IMT cut-offs for GCA, at baseline 16/18 (89%) patients had involvement of the TA and 3/18 (17%) of the AA/SA. At week 12, the IMT was above the cut-off in 11/14 (79%) in the TA (1 missing value (MV)) and in 6/14 (43%) in the AA/SA (1 MV). At week 24, the IMT was above the cut-off in 10/13 (77%) in the TA and in 6/13 (46%) in the AA/SA. Of the individual segments of the TA at baseline, 66/92 (72%) were above the diagnostic cut-off, at week 12 and 24 the proportions fell to 34/50 (68%) and 27/50 (54%) respectively. Of the individual segments of the AA/SA at baseline, 10/70 (14%) were above the diagnostic cut-off, at week 12 and 24 the proportions rose to 19/52 (37%) and 20/52 (38%) respectively. At week 4, in 7/14 (50%) patients (5 MV), IMT values of TA were higher compared to day 2/3 and in three patients, new onset AA or SA involvement was seen. Figure 1 shows the sum of standardized IMT of the TA from baseline to week 24.
Conclusion: A three day glucocorticoid pulse, followed by TCZ monotherapy leads to a gradual reduction of IMT of the TA until week 24.
This is the first study to document the usefulness and feasibility of IMT monitoring with US in GCA patients treated with TCZ.
To cite this abstract in AMA style:
Seitz L, Christ L, Scholz G, Lötscher F, Amsler J, Kollert F, Reichenbach S, Villiger P. Quantitative Ultrasound of Temporal, Axillary and Subclavian Arteries to Monitor Tocilizumab Treatment in Patients with Newly Diagnosed Giant Cell Arteritis: A 24 Week Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/quantitative-ultrasound-of-temporal-axillary-and-subclavian-arteries-to-monitor-tocilizumab-treatment-in-patients-with-newly-diagnosed-giant-cell-arteritis-a-24-week-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantitative-ultrasound-of-temporal-axillary-and-subclavian-arteries-to-monitor-tocilizumab-treatment-in-patients-with-newly-diagnosed-giant-cell-arteritis-a-24-week-analysis/