Session Information
Date: Monday, November 9, 2020
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Tocilizumab (TCZ) has low immunogenicity in patients with rheumatoid arthritis (RA).1 The risk for TCZ immunogenicity remains to be determined in patients with giant cell arteritis (GCA). TCZ administered subcutaneously every week (QW) or every other week (Q2W) plus 26-week prednisone tapering was superior to placebo (PBO) plus 26-week (PBO+26) or 52-week (PBO+52) prednisone tapering for achievement of sustained remission in patients with GCA in the 52-week, double-blind part (part 1) of the GiACTA trial.2 Part 2 was a 2-year, open-label, long-term, follow-up in which patients were treated at the investigators’ discretion; part 2 treatment could include initiation/termination of TCZ QW with or without glucocorticoids or methotrexate. The objective of this analysis was to investigate immunogenicity of TCZ QW and Q2W regimens over the course of parts 1 and 2 of GiACTA.
Methods: In parts 1 and 2 combined, anti–TCZ antibodies (ADA) and corresponding pharmacokinetic (PK) parameters were assessed in serum samples at weeks 0, 8, 24, 36, 52, 76, 100, 136, and 156 or at early withdrawal, with additional assessments in patients who interrupted blinded TCZ for ≥4 weeks in part 1 and patients who withdrew because of anaphylaxis/hypersensitivity at any time. All samples were tested sequentially by screening assay and confirmation assay to verify specificity. If the confirmation assay was positive, 2 additional tests were performed to characterize the detected ADA: a neutralizing assay to test the neutralizing potential of ADAs and an assay to determine whether the ADAs were of the IgE isotype. Proportions of patients in whom ADA developed were summarized for the safety population. ACR GCA classification criteria were fulfilled by 78% of enrolled patients. The study was conducted in accordance with the principles of the Declaration of Helsinki and received IRB approval.
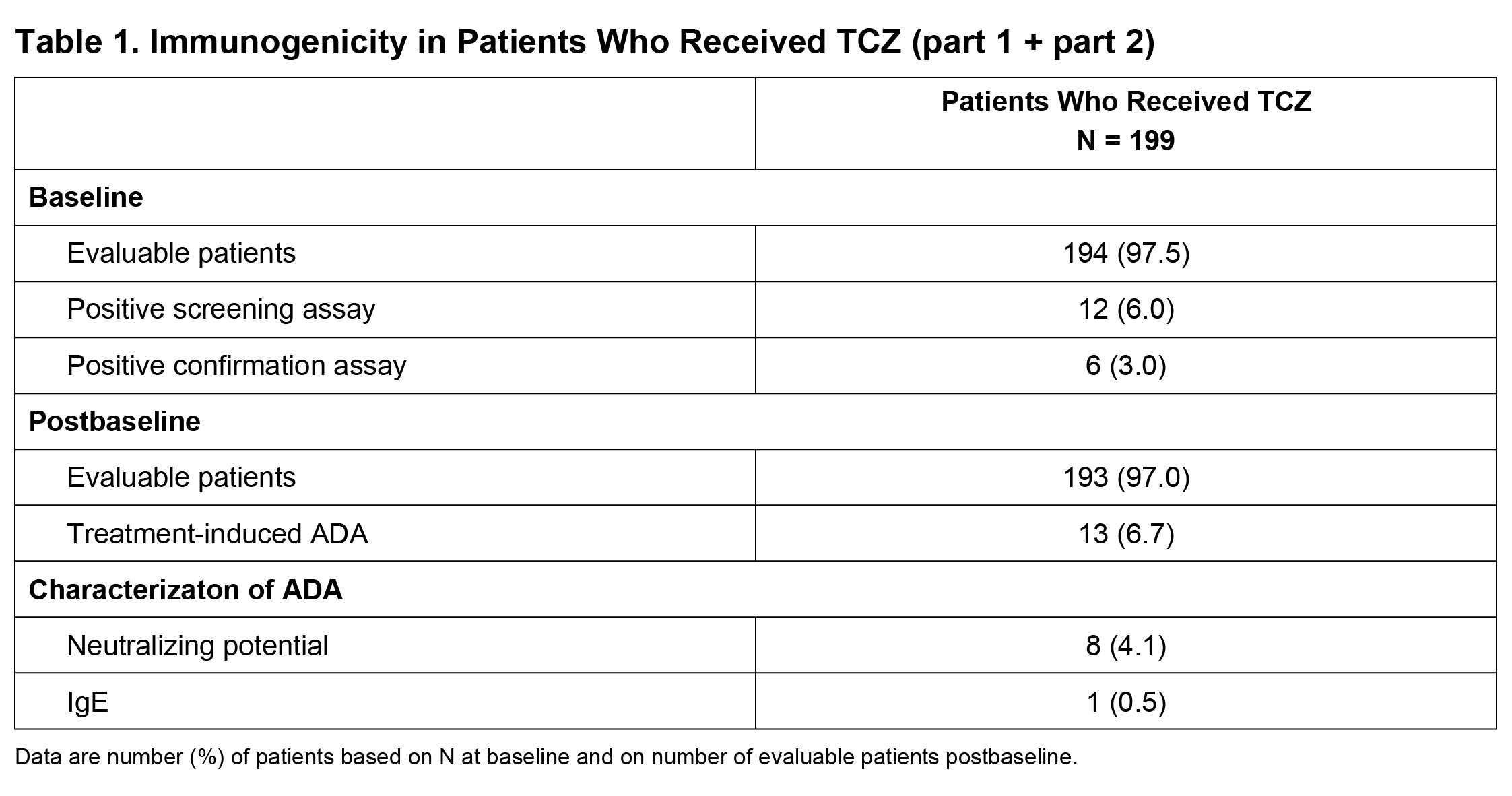
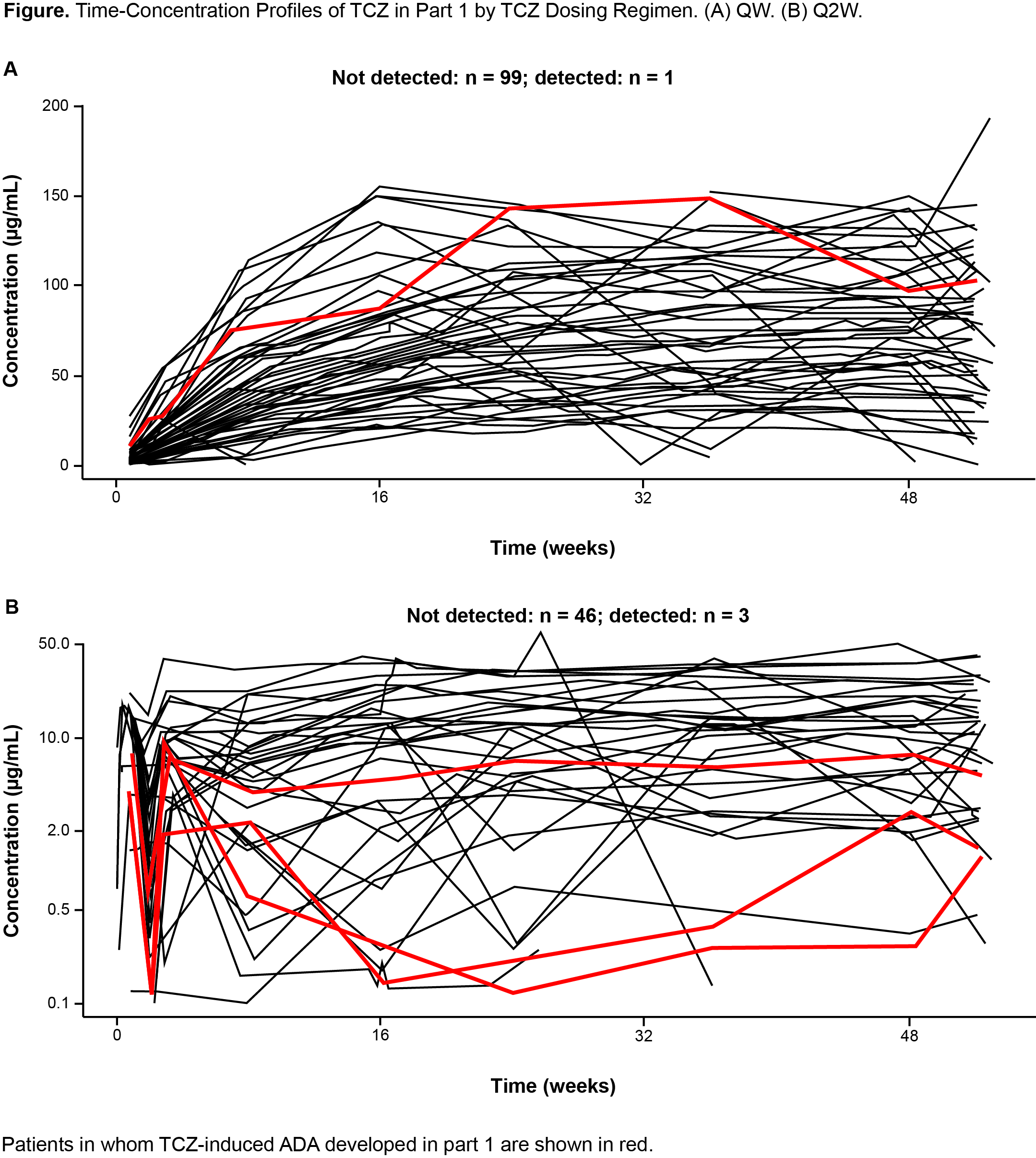
Results: Among evaluable patients (had baseline and ≥1 postbaseline ADA assessments and received ≥1 dose of study treatment) in part 1, ADA developed in 1 of 95 (1.1%) and 3 of 46 (6.5%) patients after TCZ QW and Q2W dosing, respectively. One of 49 (2.0%) and 1 of 47 (2.1%) patients in the PBO+26 and PBO+52 groups, respectively, tested positive for ADA but had not received TCZ and were considered false positives. In parts 1 and 2 combined, among 199 patients who received ≥1 dose of TCZ, 193 (97%) were evaluable (Table 1); TCZ-induced ADA developed in 13 patients (6.7%) postbaseline (4 during part 1, 9 during part 2). Of these 13 patients, 8 (4.1%) had ADA with neutralizing potential and 1 (0.5%) had IgE ADA. Most TCZ-induced ADA were transient. There was no clear impact of TCZ-induced ADA on TCZ PK (Figure 1). No patients with TCZ-induced ADA experienced anaphylaxis, hypersensitivity reactions, or injection site reactions, and none withdrew because of lack of efficacy.
Conclusion: In patients with GCA, treatment-induced ADA developed in a minority of patients and had no clear impact on TCZ PK, efficacy, or safety. The immunogenicity of subcutaneous TCZ treatment was low, consistent with that observed in patients with RA.
References: 1. Burmester GR et al. Ann Rheum Dis 2017;76:1078-85; 2. Stone JH et al. N Engl J Med 2017;377:317-28.
To cite this abstract in AMA style:
Stone J, Mallalieu N, Bao M. Low Immunogenicity in Patients with Giant Cell Arteritis Treated with Tocilizumab: 3-Year Results from the Randomized Controlled Portion and the Open-Label Follow-Up of a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/low-immunogenicity-in-patients-with-giant-cell-arteritis-treated-with-tocilizumab-3-year-results-from-the-randomized-controlled-portion-and-the-open-label-follow-up-of-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/low-immunogenicity-in-patients-with-giant-cell-arteritis-treated-with-tocilizumab-3-year-results-from-the-randomized-controlled-portion-and-the-open-label-follow-up-of-a-phase-3-trial/