Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: SLE is a heterogeneous autoimmune disease with clinical manifestations across multiple organ systems. In the phase 3 TULIP-1 and TULIP-2 trials, anifrolumab treatment resulted in greater percentages of patients with SLE achieving a BILAG-based Composite Lupus Assessment (BICLA) response vs placebo at Week 52.1,2 We further evaluated the effects of anifrolumab on organ domain–specific SLE disease activity using pooled data from TULIP-1 and TULIP-2.
Methods: TULIP-1 and TULIP-2 were 52-week, randomized, double-blind, placebo-controlled trials that evaluated the efficacy and safety of anifrolumab (300 mg IV every 4 weeks for 48 weeks) in patients with moderately to severely active SLE despite standard-of-care treatment. Using pooled data, we compared BILAG-2004 and SLEDAI-2K responses across individual organ domains in patients receiving anifrolumab vs placebo. Organ improvement was defined as a reduction in baseline BILAG-2004 or SLEDAI-2K organ domain scores and was assessed at Week 52. Tender and swollen joint counts were recorded, and an improvement was defined as a reduction of ≥50% from baseline in counts of both tender and swollen joints.
Results: In total, 360 patients received anifrolumab and 366 received placebo. The percentages of patients with BILAG-2004 A or B and SLEDAI-2K organ domain scores at baseline were generally similar between treatment groups across all organ domains. The most frequently affected organ domains at baseline were mucocutaneous and musculoskeletal by both BILAG-2004 and SLEDAI-2K, and immunology by SLEDAI-2K. Baseline BILAG-2004 A or B scores occurred less frequently in the cardiorespiratory, constitutional, renal, neuropsychiatric, gastrointestinal, hematologic, and ophthalmic domains, and baseline SLEDAI-2K activity was less frequent in the hematologic, fever, vascular, renal, cardiorespiratory, and central nervous system domains. At Week 52, a greater number of patients treated with anifrolumab vs placebo had improvements in the mucocutaneous and musculoskeletal domains by both BILAG-2004 and SLEDAI-2K (Figures 1 and 2). Improvements were also observed in the majority of less frequently affected domains (Figures 1 and 2). Higher percentages of patients treated with anifrolumab vs placebo achieved ≥50% reductions in baseline tender and swollen joint counts at Weeks 36, 44, 48, and 52 in those with baseline tender and swollen joint counts of ≥6 and ≥8 (Figure 3).
Conclusion: Higher percentages of patients treated with anifrolumab compared with placebo had improved BILAG-2004 and SLEDAI-2K domain scores at Week 52 in the 2 most frequently affected domains (mucocutaneous and musculoskeletal) and in less frequently affected domains, as well as reductions in arthritis scores. These data provide evidence of the benefit of anifrolumab across multiple organ domains in patients with active SLE.
References
- Furie RA. Lancet Rheumatol. 2019;1:e208–19.
- Morand EF. N Engl J Med. 2020;382:211–21.
Writing assistance by Rebecca Jones, PhD (JK Associates, Inc., a Fishawack Health Company).
This study was sponsored by AstraZeneca.
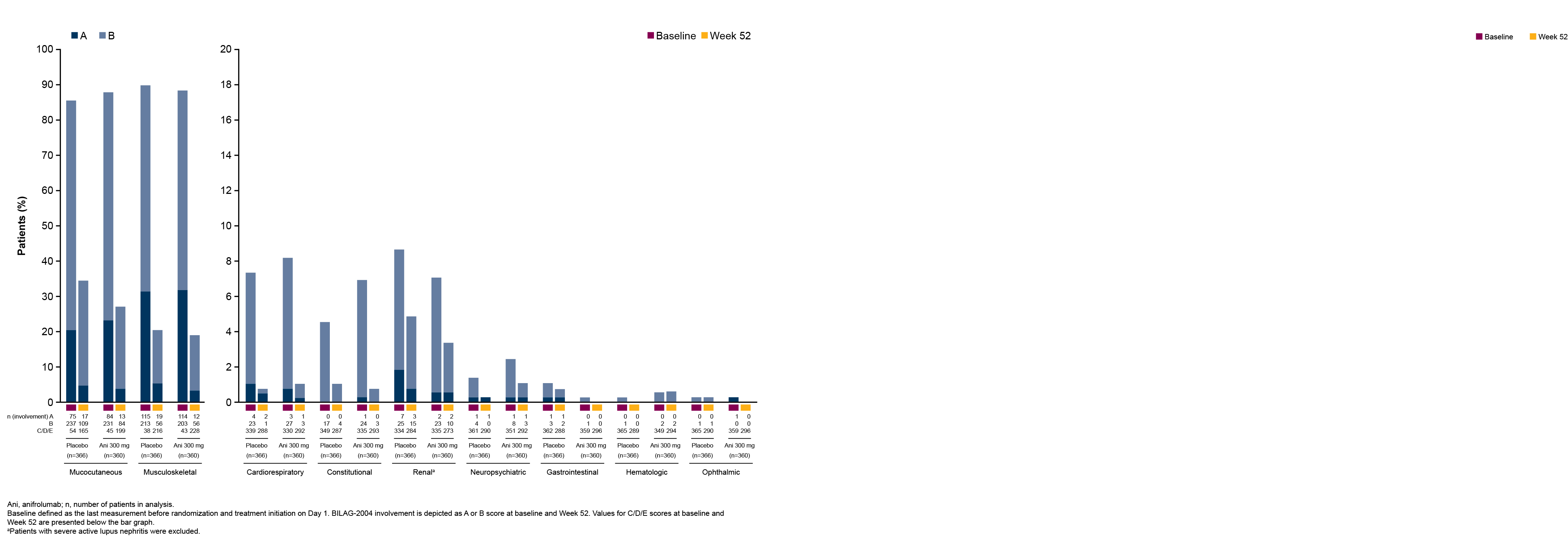 Figure 1. BILAG-2004 Organ Involvement at Baseline and Week 52 for Patients With SLE Receiving Anifrolumab or Placebo in Pooled Data From the TULIP-1 and TULIP-2 Trials
Figure 1. BILAG-2004 Organ Involvement at Baseline and Week 52 for Patients With SLE Receiving Anifrolumab or Placebo in Pooled Data From the TULIP-1 and TULIP-2 Trials
 Figure 2. SLEDAI-2K Organ Improvement at Week 52 for Patients With SLE Receiving Anifrolumab or Placebo in Pooled Data From the TULIP-1 and TULIP-2 Trials
Figure 2. SLEDAI-2K Organ Improvement at Week 52 for Patients With SLE Receiving Anifrolumab or Placebo in Pooled Data From the TULIP-1 and TULIP-2 Trials
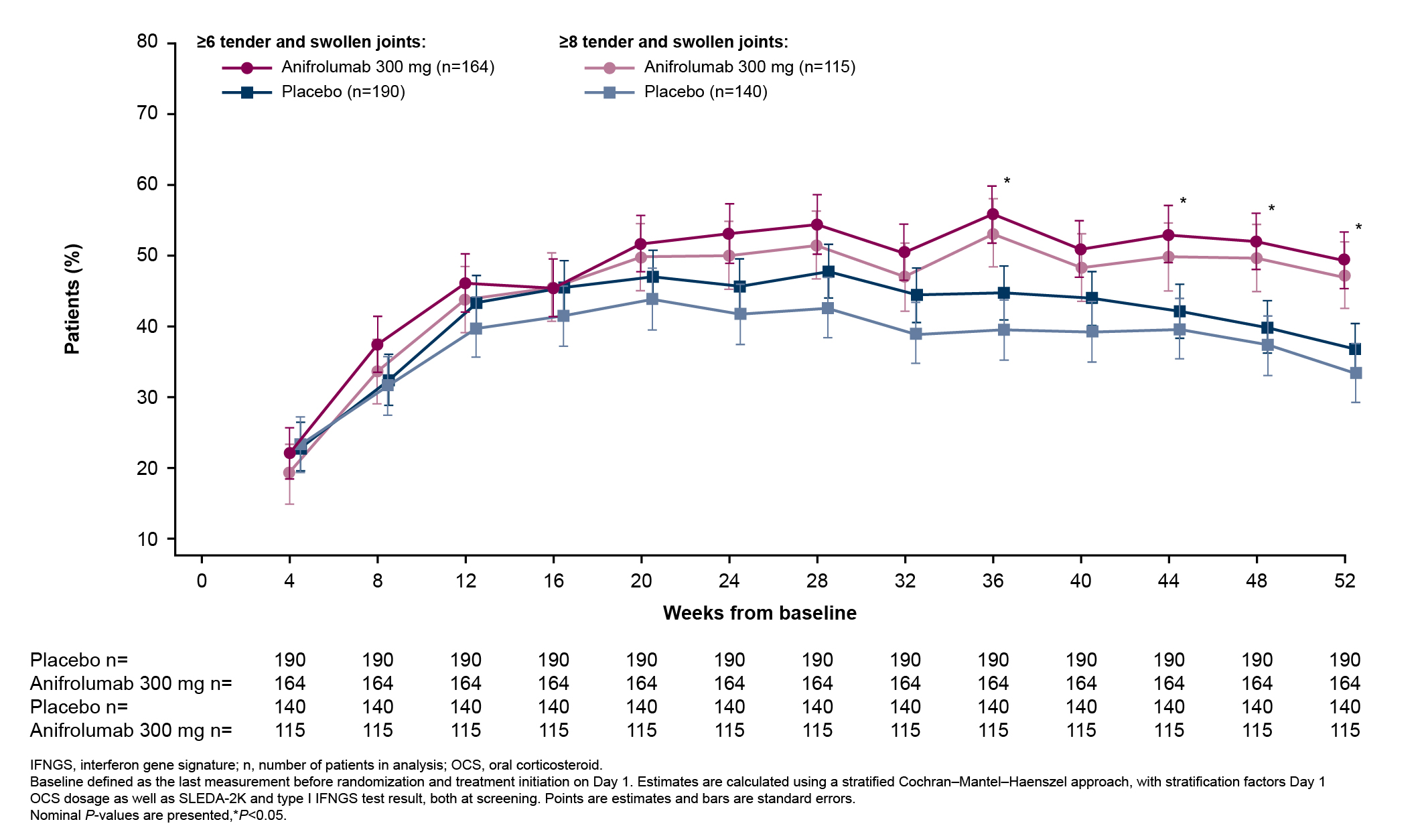 Figure 3. Percentage of Patients Who Achieved ≥50% Reductions in Baseline Tender and Swollen Joint Counts Over Time in Patients With ≥6 and ≥8 Tender and Swollen Joints at Baseline in Pooled Data From the TULIP-1 and TULIP-2 Trials
Figure 3. Percentage of Patients Who Achieved ≥50% Reductions in Baseline Tender and Swollen Joint Counts Over Time in Patients With ≥6 and ≥8 Tender and Swollen Joints at Baseline in Pooled Data From the TULIP-1 and TULIP-2 Trials
To cite this abstract in AMA style:
Morand E, Furie R, Bruce I, Vital E, Dall'Era M, Maho E, Pineda L, Tummala R. Comprehensive Efficacy of Anifrolumab Across Organ Domains in Patients with Active SLE: Pooled Data from 2 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comprehensive-efficacy-of-anifrolumab-across-organ-domains-in-patients-with-active-sle-pooled-data-from-2-phase-3-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comprehensive-efficacy-of-anifrolumab-across-organ-domains-in-patients-with-active-sle-pooled-data-from-2-phase-3-trials/
