Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Both IL-12 and IL-23 have been implicated in the pathogenesis of SLE. In a phase 2 study, treatment with the anti-IL-12/23 p40 monoclonal antibody ustekinumab (UST) resulted in greater improvement in several SLE disease measures through week (wk) 24 compared with placebo (PBO)1, and efficacy was maintained through 1 year.2 Here we report long-term safety and efficacy of ustekinumab through 2 years in patients (pts) with active SLE.
Methods: This was a PBO-controlled phase 2 study in 102 pts with seropositive active SLE (defined by SLICC criteria; SLEDAI score ≥6; ≥1 BILAG A and/or ≥2 BILAG B scores). Pts were randomized (3:2) to UST (~ 6 mg/kg single IV infusion, then 90 mg SC q8w at wk8) or PBO, both added to standard background therapy. Patients receiving PBO crossed over to UST (90 mg SC q8w) at wk24. The majority of pts were eligible to enter a voluntary open-label study extension after wk40; the final UST administration was at wk104. The primary endpoint was SLE Responder Index (SRI)-4 response at wk24. Treatment failure rules and nonresponder imputation were used through wks 24 & 48;1,2 observed data are reported from week 48 – week 112. Disease flare was defined as ≥1 new BILAG A or ≥2 new BILAG B scores. Safety was assessed through wk120.
Results: 102 pts were randomized at wk0 (UST, n=60; PBO, n=42). The primary endpoint (SRI-4 response rate at wk24) was met,1 and efficacy was sustained at 1 year.2 46 pts entered the extension (UST, n=29; PBO → UST, n=17), 26 pts at wk48, and 20 pts at wk56. SRI-4 response rates were maintained during the extension and were 79% in the UST group and 92% in the PBO → UST group at wk112. In both groups, response rates in joint and skin measures were maintained from wk48 through wk112 (Figures). At wk112, 92% in both the UST and PBO → UST groups had ≥4-point improvement from baseline in SLEDAI-2K score, 79% and 93% had ≥30% improvement from baseline in PGA, 86% and 91% had ≥50% improvement in active joint (pain and inflammation) count, and 79% and 100% had ≥50% improvement in CLASI score. During the extension period, the incidence per 100 pt-years of a BILAG 1A/2B flare was 0 in the PBO → UST group and 1.95 in the UST group. Improvements ≥2.5 points (Minimal Clinically Important Difference [MCID]) in SF-36 PCS scores were observed and maintained from baseline through wk72 (UST group) and at most timepoints from wk40 to wk112 (PBO → UST). No deaths, malignancies, opportunistic infections, or tuberculosis cases occurred through week 120. Of 93 pts who received ≥1 dose of UST through wk120, 17 (18%) had a serious AE. Safety events were consistent with the known UST safety profile.
Conclusion: UST provided sustained clinical benefit in global and organ-specific SLE-activity measures through 2 years with safety results consistent with the established profile.
References:
- van Vollenhoven RF et al. Lancet 2018;392:1330.
- Arthritis Rheumatol. 2019 Nov 25. doi: 10.1002/art.41179
 Figure 1. Proportions of patients with SRI-4 (A), SLEDAI-2K (B), and PGA (C) responses through week 112. SRI-4 = SLEDAI-2K responder index, defined as ≥ 4-point reduction in SLEDAI-2K total score, no new BILAG A and no more than 1 new BILAG B domain score and no worsening ( < 10% increase) from baseline in the PGA. SLEDAI-2K = Systemic Lupus Erythematosus Disease Activity Index 2000 (response defined as ≥ 4-point improvement from baseline). PGA = Physician’s Global Assessment (response defined as ≥ 30% improvement from baseline). The study protocol was amended to add the extension period after the interim analysis, and some patients had already completed or discontinued from the main study. Patients participating in the study extension entered either at Week 48 or Week 56.
Figure 1. Proportions of patients with SRI-4 (A), SLEDAI-2K (B), and PGA (C) responses through week 112. SRI-4 = SLEDAI-2K responder index, defined as ≥ 4-point reduction in SLEDAI-2K total score, no new BILAG A and no more than 1 new BILAG B domain score and no worsening ( < 10% increase) from baseline in the PGA. SLEDAI-2K = Systemic Lupus Erythematosus Disease Activity Index 2000 (response defined as ≥ 4-point improvement from baseline). PGA = Physician’s Global Assessment (response defined as ≥ 30% improvement from baseline). The study protocol was amended to add the extension period after the interim analysis, and some patients had already completed or discontinued from the main study. Patients participating in the study extension entered either at Week 48 or Week 56.
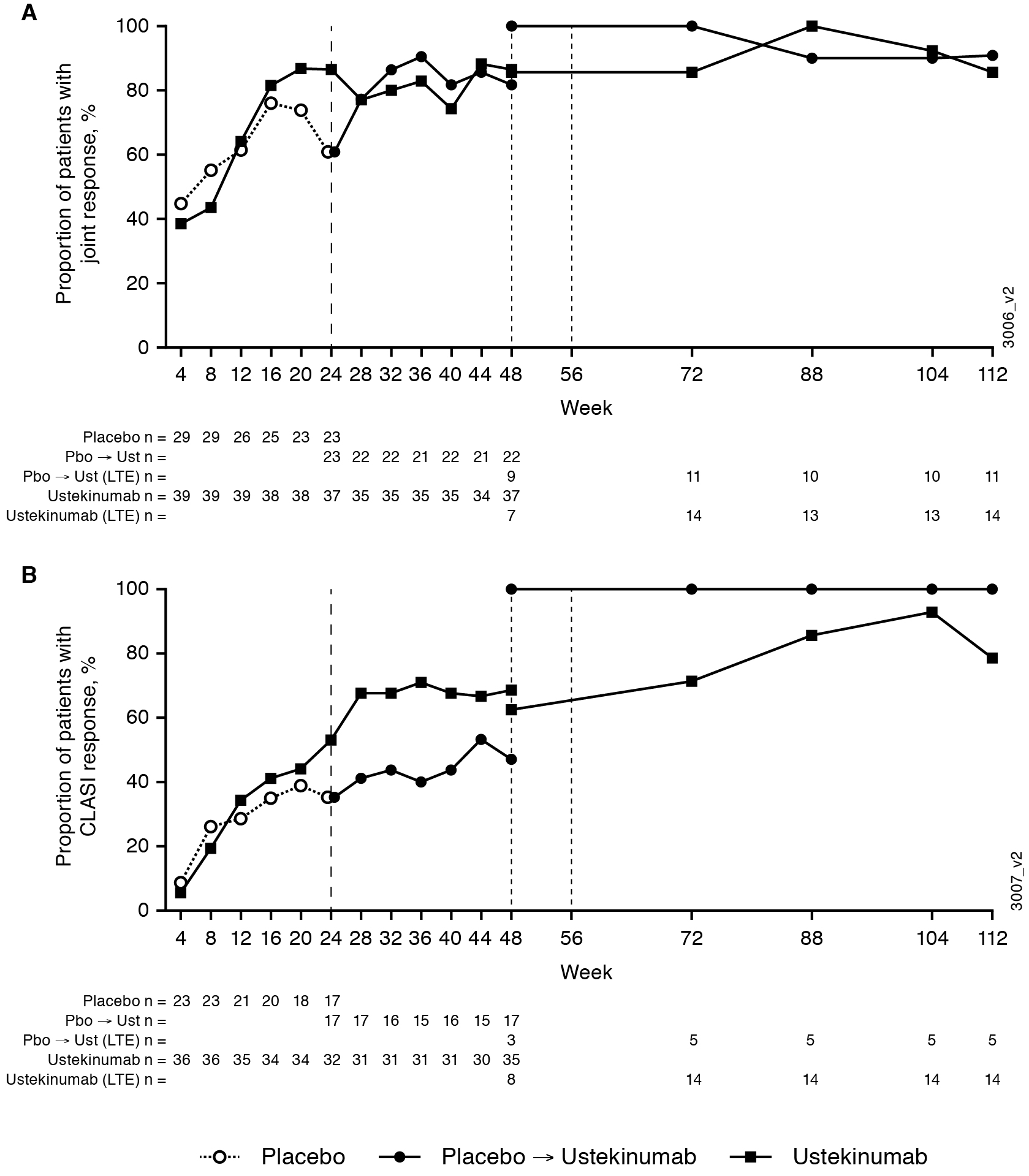 Figure 2. Proportions of patients with joint (A) and CLASI (B) responses. The study protocol was amended to add the extension period after the interim analysis, and some patients had already completed or discontinued from the main study. Patients participating in the study extension entered either at Week 48 or Week 56. Joint response (defined as ≥ 50% improvement from baseline in the number of joints with pain and inflammation [active joints] in patients with ≥4 active joints at baseline). CLASI response (defined as ≥ 50% improvement from baseline in patients with a baseline CLASI activity score ≥4).
Figure 2. Proportions of patients with joint (A) and CLASI (B) responses. The study protocol was amended to add the extension period after the interim analysis, and some patients had already completed or discontinued from the main study. Patients participating in the study extension entered either at Week 48 or Week 56. Joint response (defined as ≥ 50% improvement from baseline in the number of joints with pain and inflammation [active joints] in patients with ≥4 active joints at baseline). CLASI response (defined as ≥ 50% improvement from baseline in patients with a baseline CLASI activity score ≥4).
To cite this abstract in AMA style:
van Vollenhoven R, Hahn B, Tsokos G, Lipsky P, Gordon R, Fei K, Lo K, Chevrier M, Zuraw Q, Berry P, Karyekar C, Rose S. Maintenance of Efficacy and Safety and Reduction of BILAG Flares with Ustekinumab, an Interleukin-12/23 Inhibitor, in Patients with Active Systemic Lupus Erythematosus: 2-Year Results of a Phase 2, Randomized Placebo-Controlled, Crossover Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/maintenance-of-efficacy-and-safety-and-reduction-of-bilag-flares-with-ustekinumab-an-interleukin-12-23-inhibitor-in-patients-with-active-systemic-lupus-erythematosus-2-year-results-of-a-phase/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maintenance-of-efficacy-and-safety-and-reduction-of-bilag-flares-with-ustekinumab-an-interleukin-12-23-inhibitor-in-patients-with-active-systemic-lupus-erythematosus-2-year-results-of-a-phase/

