Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Definitions of disease activity are not standardized in Takayasu’s arteritis (TAK), which can lead to difficulty in determining whether a patient should be enrolled into a randomized controlled trial (RCT). FDG-PET can complement clinical assessment of disease activity. Two prior RCTs in TAK (AGATA, North American study of abatacept1 and TAKT, Japanese study of tocilizumab2) used different definitions of disease activity. Neither trial considered FDG-PET findings in these definitions.
The objectives of this study were to: 1) Evaluate physician agreement about patient enrollment based on study inclusion criteria from AGATA and TAKT and 2) Determine if vascular FDG-PET impacts decision-making about trial inclusion.
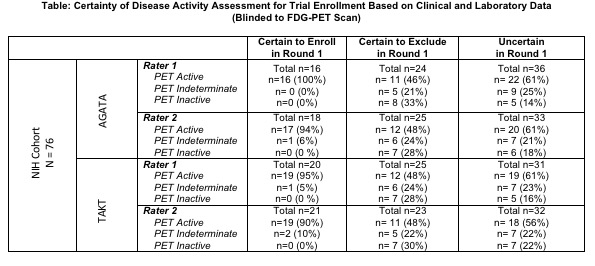
Methods: Patients with TAK were recruited from an observational cohort. Each patient underwent clinical assessment and an FDG-PET-CT scan during a single study visit. A nuclear medicine physician reviewed all PET scans, blinded to clinical data, and interpreted the scans as active, indeterminate, or inactive. Two rheumatologists independently rated whether each patient met inclusion criteria for AGATA1 and/or TAKT2. In Round 1, determination of disease activity was based on clinical and laboratory data only. In Round 2, determination of disease activity was based on the same data plus PET scan results. In both rounds, each reviewer recorded certainty about the decision to enroll/exclude a patient. Inter-rater reliability (IRR) was calculated using Fleiss’ Kappa.
Results: 76 patients with TAK were evaluated. Using AGATA enrollment criteria, the raters agreed to enroll 31 patients (41%), exclude 33 patients (43%), and disagreed for 12 patients (16%). Using TAKT enrollment criteria, the raters agreed to enroll 34 patients (45%), exclude 29 patients (38%), and disagreed for 13 patients (17%). Disagreement was frequently driven by fatigue/malaise and/or elevated acute phase reactants, and raters were uncertain in 92% of these cases. Availability of PET scan results significantly improved agreement between raters (Round 1 IRR= 0.68 [95% CI (0.67-0.69)]; Round 2 IRR=0.88 [CI 0.87-0.89]; p< 0.01).
Of patients whom the rater was certain in Round 1 to enroll, no patient had an inactive PET. Conversely, of patients whom the rater was certain to exclude, approximately half of these patients had abnormal subclinical PET activity (TABLE). For patients whom the rater noted uncertainty about enrollment in Round 1, certainty improved in both raters once PET data was incorporated [Rater 1: 56% to 86% certainty, Rater 2: 57% to 87% certainty].
Conclusion: Physicians are frequently uncertain about disease activity assessment in TAK. This uncertainty leads to disagreement about which patients meet RCT requirements for enrollment. Consideration of FDG-PET findings in addition to clinical assessment improves confidence about disease activity assessment and significantly reduces disagreement about trial eligibility. Incorporation of PET findings into the definition of disease activity identifies a substantial number of patients with subclinical inflammation, which could increase the number of eligible participants for treatment trials in TAK.
References:
- Langford CA, et al A&R 2017
- Nakaoka Y, et al ARD 2018
To cite this abstract in AMA style:
Quinn K, Ahlman M, Rose E, Merkel P, Grayson P. Enrichment of Clinical Trial Recruitment Using Advanced Molecular Imaging in Takayasu’s Arteritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/enrichment-of-clinical-trial-recruitment-using-advanced-molecular-imaging-in-takayasus-arteritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/enrichment-of-clinical-trial-recruitment-using-advanced-molecular-imaging-in-takayasus-arteritis/