Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Enthesitis is a common clinical feature of psoriatic arthritis (PsA). There is limited evidence on the effect of treatment on enthesitis. Our purpose was to study the effectiveness of conventional and targeted (c and t) DMARDs in treating clinical enthesitis in an observational PsA cohort.
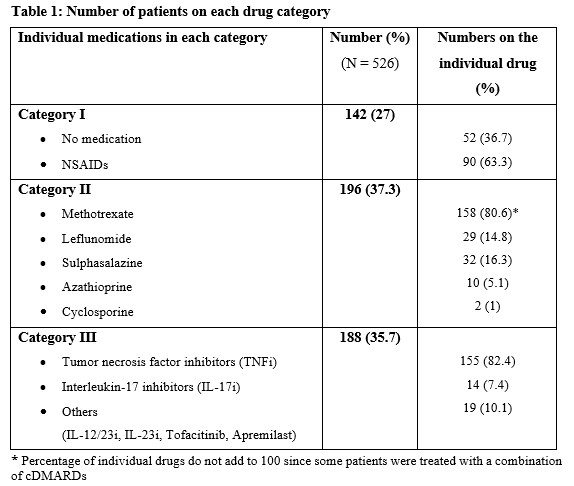
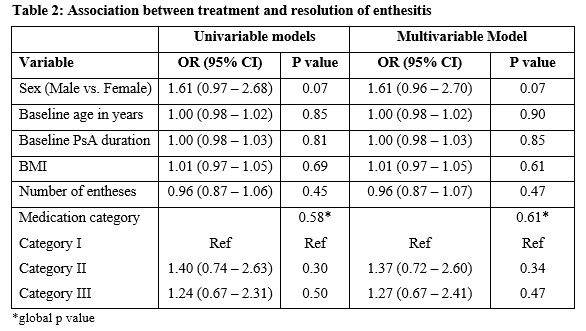
Methods: Patients enrolled into a large PsA cohort from 1 January 2000 to 6 May 2020, with clinical enthesitis were included in this study. Enthesitis was defined as tenderness over at least 1 of the 29 sites described by validated enthesitis scoring indices, including spondyloarthritis research consortium of Canada (SPARCC) enthesitis index, Leeds enthesitis index (LEI), and Maastricht ankylosing spondylitis enthesitis score (MASES). Baseline medications, including non-steroidal anti-inflammatory drugs (NSAIDs) and/or c/t DMARDs at the diagnosis of enthesitis, were recorded for each patient. Complete resolution of enthesitis at 12 months was the primary outcome. The pharmacological treatment prescribed was divided into the following mutually exclusive ordinal categories: I- No treatment/NSAIDs; II- cDMARDs±NSAIDs, without tDMARD; III- tDMARDs±cDMARDs/NSAIDs. Univariable and multivariable logistic regression models were created to determine the association between medication category and complete resolution of enthesitis after controlling for age, sex, body mass index (BMI), PsA duration and baseline enthesitis score. A global p-value was calculated to evaluate the overall effect of medication categories on enthesitis resolution. The results are expressed as odds ratios (OR) with a 95% confidence interval (CI).
Results: Of the 1270 patients, 628 (49.4%) had enthesitis. After excluding 102 patients for inadequate follow-up data, 526 patients (51.7% males) with a mean age of 49.02 years (sd=13.12) years were included. The mean tender entheses count was 2.13 (sd=2.16). The mean duration of PsA at baseline was 10.74 years (sd=10.73), and the mean BMI was 29.13 (sd=6.25). The proportion of patients treated with each category of medications is presented in Table 1. Complete resolution of enthesitis was noted in 453 (86%) patients, within a mean of 8.9 months. The results of the regression analysis are shown in Table 2. The global p-value for medication categories was not significant in univariate and multivariable models. The models showed a trend for males to achieve complete resolution of enthesitis regardless of the medication category (OR 1.71; 95% CI 0.96-2.70; p=0.07).
Conclusion: In an observational setting resolution of clinical enthesitis occurs regardless of the treatment used. Future effectiveness studies may require evaluating patients with severe enthesitis using advanced imaging.
To cite this abstract in AMA style:
Mathew A, Sutton M, Pereira D, Chandran V, Gladman D. Efficacy of Disease Modifying Anti-Rheumatic Drugs for Enthesitis in a Prospective Longitudinal Psoriatic Arthritis Cohort [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-disease-modifying-anti-rheumatic-drugs-for-enthesitis-in-a-prospective-longitudinal-psoriatic-arthritis-cohort/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-disease-modifying-anti-rheumatic-drugs-for-enthesitis-in-a-prospective-longitudinal-psoriatic-arthritis-cohort/