Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The concept of prevention of psoriatic arthritis (PsA) has gained increased interest given the physical limitation and poor quality-of-life experienced by PsA patients coupled with low remission rates observed with the use of currently available therapies. The transition from psoriasis to PsA offers a unique window of opportunity to identify individuals at increased risk for PsA, to study and implement preventive strategies. Herein, we propose terminology to define specific subgroups during the pre-clinical and early clinical phases of PsA to facilitate and harmonize recruitment for research studies.
Methods: We conducted a 3-round online Delphi exercise including international experts in psoriatic disease. Experts were recruited from the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network (PPACMAN). Participants were asked to vote on and rank their preferred (a) terms and (b) definitions to describe pre-clinical PsA populations. Consensus for multiple choice and ranking questions was defined a priori as ≥ 70%. For questions based on a visual analogue scale, items were retained if the median was > 70. Delphi items that had < 15% of the votes or the median was < 15 were removed. If consensus was not reached, the question was carried through to the next round.
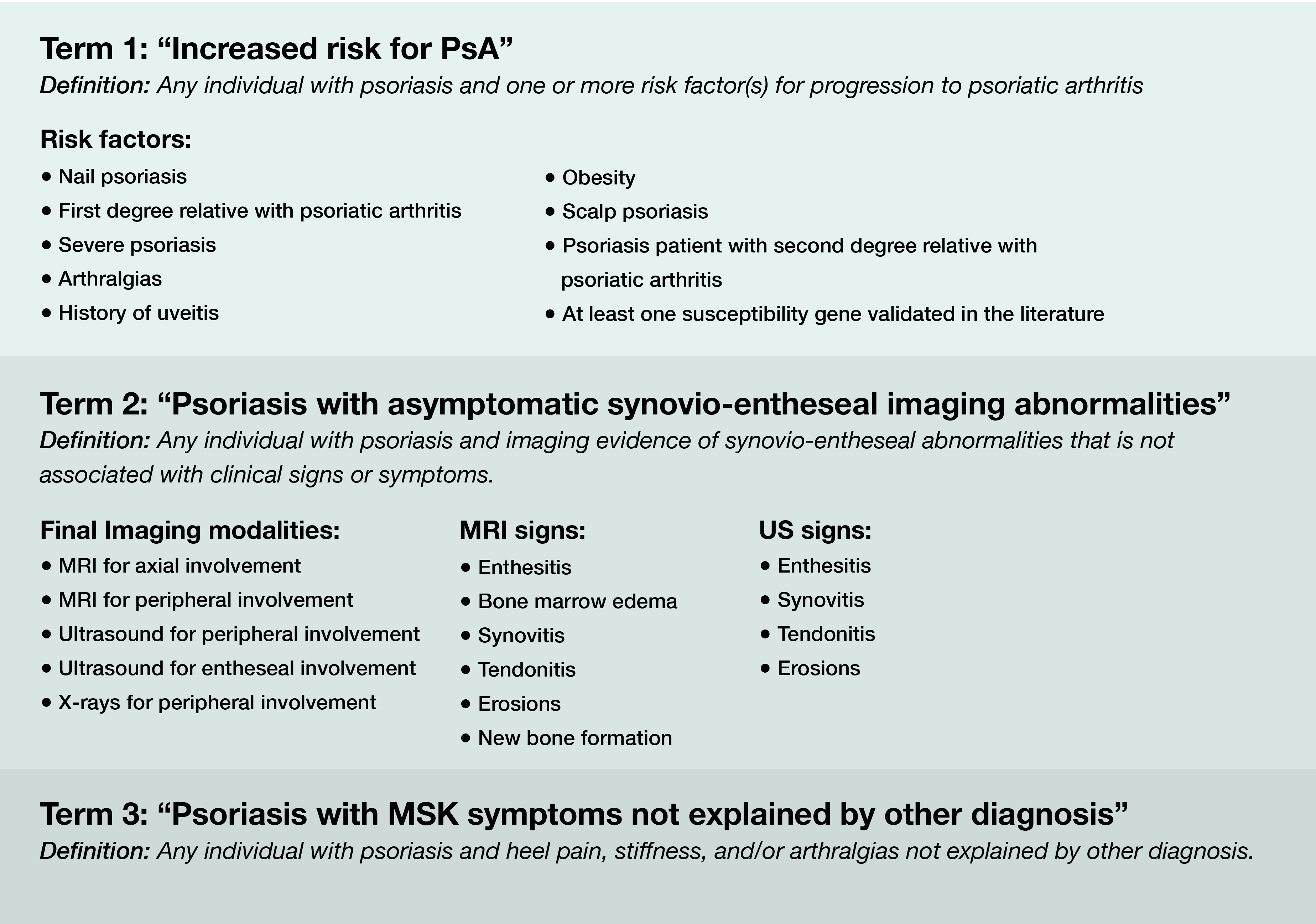
Results: A total of 29, 33, and 35 experts participated in the first, second and third Delphi round, respectively. Consensus was met for the following terms and associated definitions: “Increased risk for PsA”, “Psoriasis with asymptomatic synovio-entheseal imaging abnormalities”, and “Psoriasis with musculoskeletal symptoms not explained by other diagnosis” (Figure). Risk factors for progression to PsA that met consensus included obesity, presence of arthralgia, severe psoriasis, history of uveitis, nail psoriasis, scalp psoriasis, and first-degree relative with PsA. Specific imaging modalities to evaluate these populations were also included in the exercises and consensus was achieved.
Conclusion: In these Delphi exercises, there was agreement on three terms and definitions that characterize the psoriasis-to-PsA transition that can to be used for research purposes. The adoption of standardized nomenclature and common definitions for research in this area should improve the communication of ideas in the field and help to enroll well-defined, homogenous cohorts of psoriasis patients, for comparison across future studies. These terms and definitions may evolve as increasing evidence regarding the molecular, clinical, and imaging features of the psoriasis-to-PsA continuum emerges.
To cite this abstract in AMA style:
Haberman R, Perez-Chada L, Chandran V, Rosen C, Ritchlin C, Eder L, Mease P, Reddy S, Ogdie A, Merola J, Scher J. A Delphi Consensus Study to Standardize Terminology for the Pre-clinical Phase of Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-delphi-consensus-study-to-standardize-terminology-for-the-pre-clinical-phase-of-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-delphi-consensus-study-to-standardize-terminology-for-the-pre-clinical-phase-of-psoriatic-arthritis/