Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Date: Saturday, May 2, 2020
Title: Poster Session 3
Session Type: ACR Abstract Session
Session Time: 4:15PM-5:15PM
Background/Purpose: Amplified Musculoskeletal Pain Syndrome (AMPS), a chronic pain syndrome with excessive musculoskeletal pain without a primary organic etiology, has a high prevalence in adolescents. The current multimodal approach to the treatment of AMPS is time intensive. Investigation of alternative strategies is needed. Despite being an effective and safe modality for acute procedural pain in adolescents, immersive Virtual Reality (VR) has not been evaluated for chronic pain in adolescents. The primary aim of this pilot trial was to assess the feasibility and acceptability of VR for pain management in adolescents with AMPS.
Methods: We conducted a pilot randomized controlled trial in adolescents (13-17 years) with AMPS attending the pediatric rheumatology clinic of an urban children’s hospital from December 2018-August 2019, with a target recruitment of 30 participants. Patients with comorbidities explaining the pain or contraindications to VR use were excluded. The intervention group used an immersive VR application ‘Happy Place’ (© Mimerse-AB) on an Oculus GO (© Facebook Technologies, LLC). The control group viewed the 2D version of ‘Happy Place’ on an iPad (© Apple Inc. Cupertino CA). The interventions were a single 10-minute session in both groups. The primary outcomes were recruitment rates for feasibility and an acceptability questionnaire. The secondary outcomes were validated scales for pain intensity, pain catastrophizing and self-efficacy to manage pain. These were collected pre- and post-intervention and 2 days later.
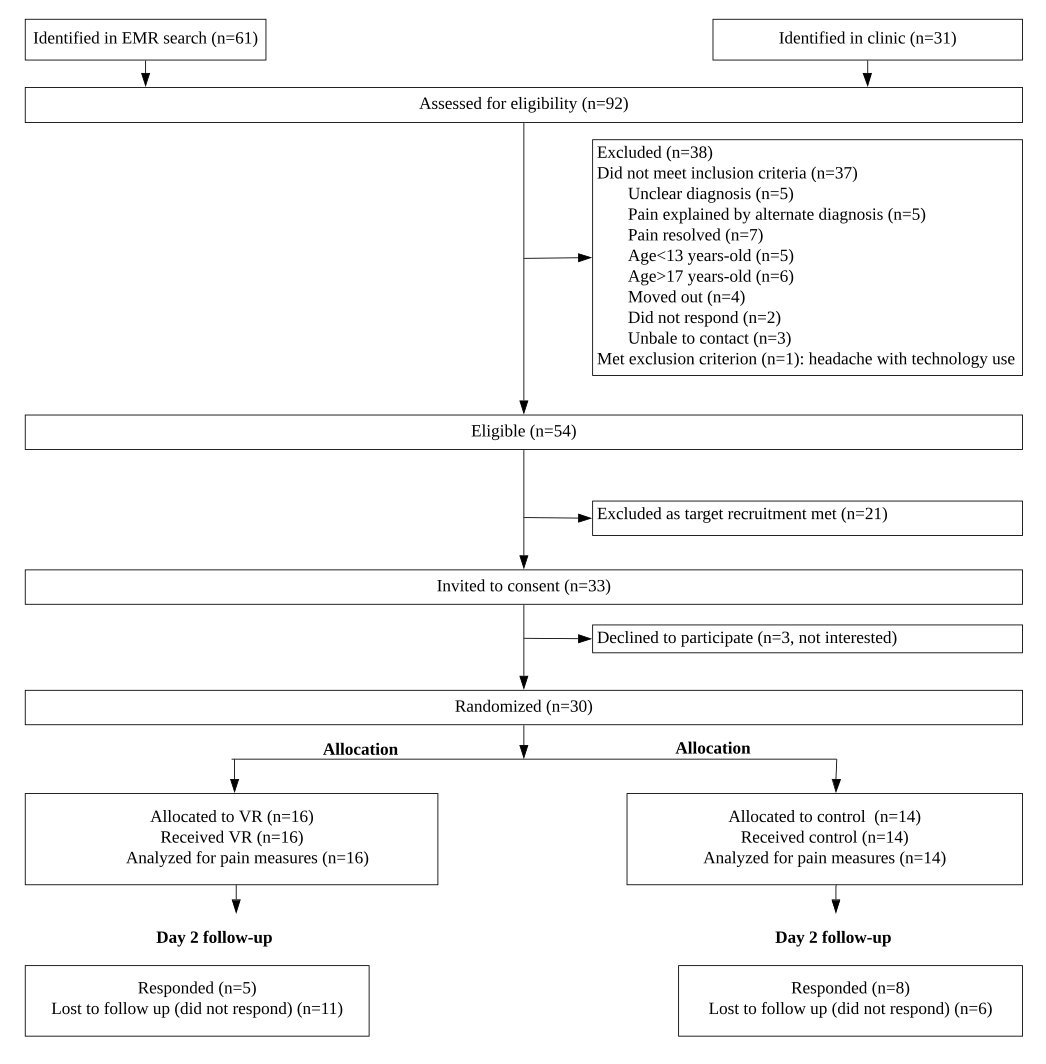
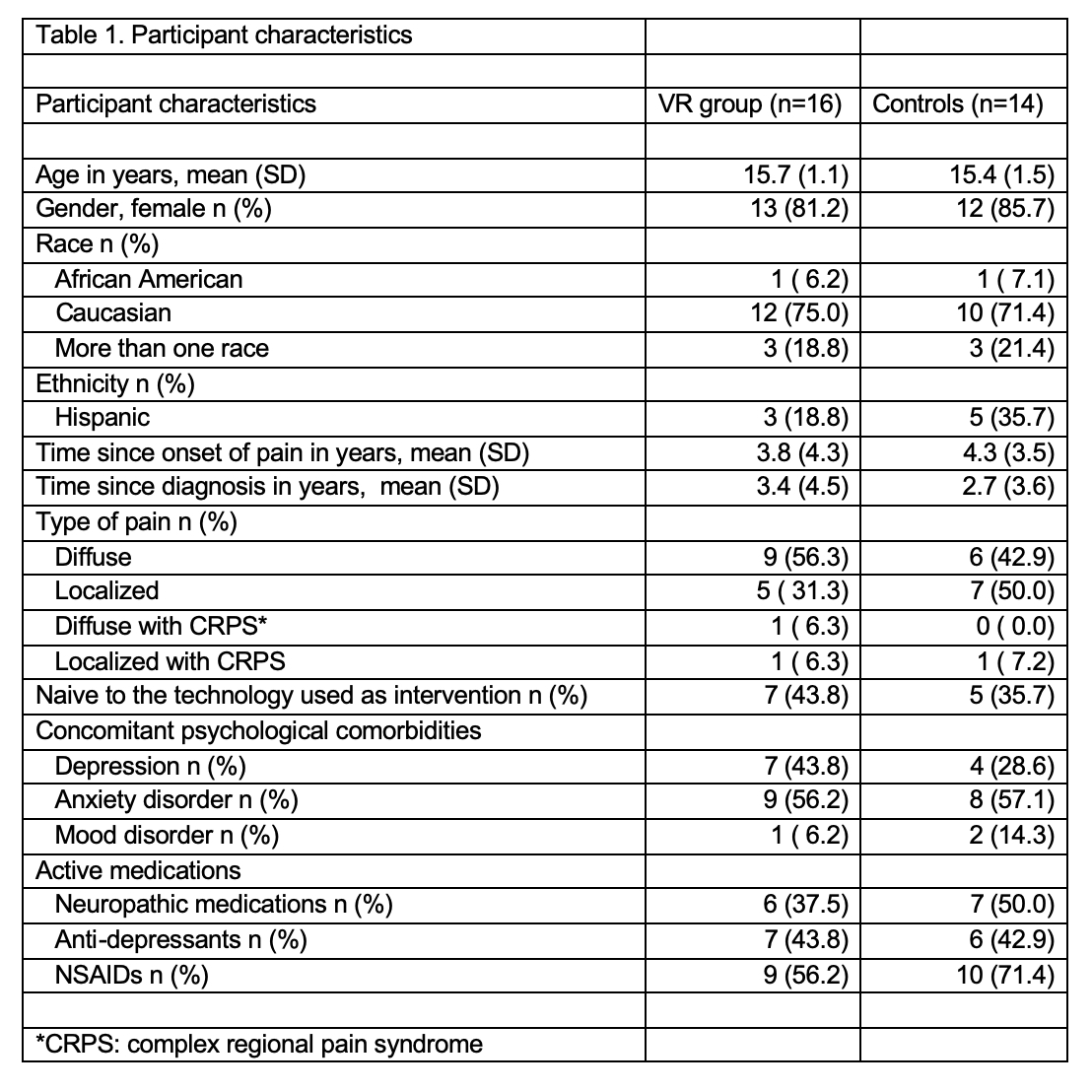
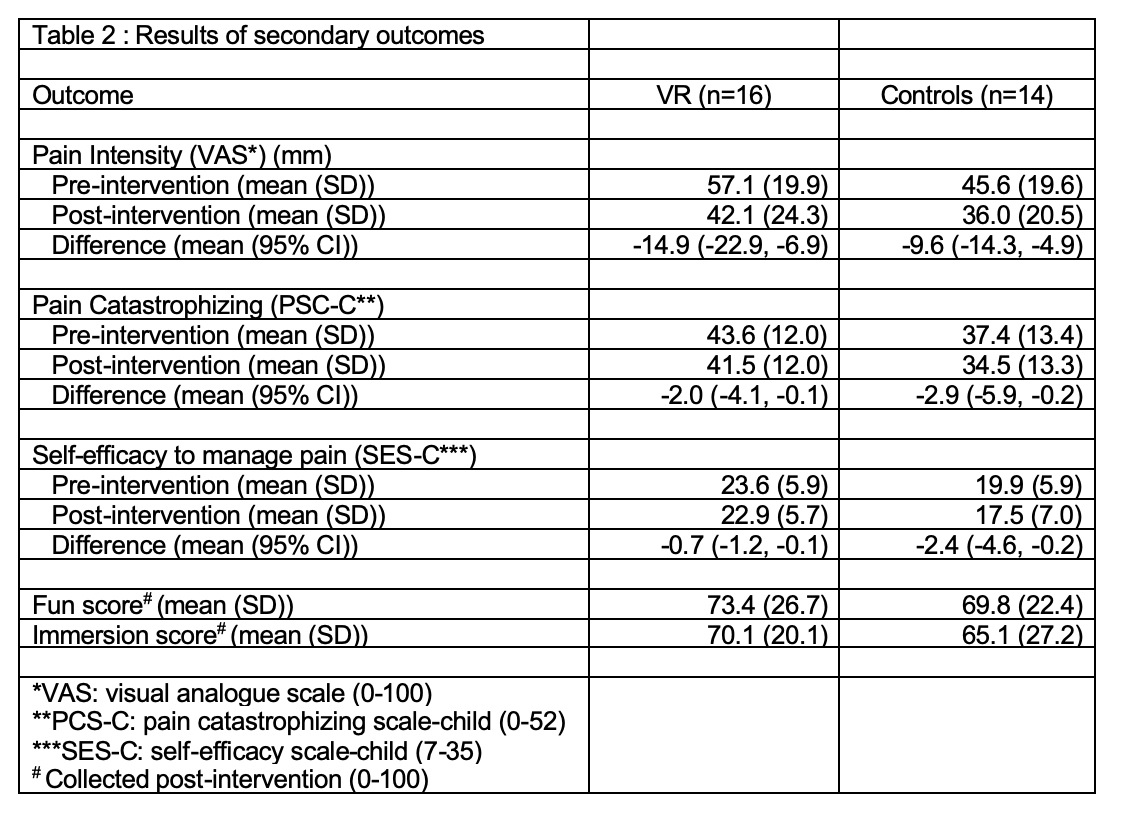
Results: Of the 92 screened patients, 54 (59%) were found eligible (Figure 1). Target recruitment was met when 30 (91%) of the 33 approached participants consented. All participants (VR = 16, control = 14, mean age = 15.6 years; 83% female) successfully completed the interventions without any adverse events (Table 1). VR group reported higher immersion and more fun than controls. The response rate for completion of questionnaires was 100% in both groups on the study day and 31% in VR group and 57% in the control group on follow-up day 2. Overall 70% participants and 93% parents expressed willingness to participate in multiple sessions in the future. The mean pain intensity reduction after treatment was 14.9 (95% CI -22.9, -6.9) in the VR group and 9.6 (95% CI -14.3, -4.9) in the controls on a visual analog scale of 0-100 (Table 2). A total of 11 (69%) participants in the VR group achieved a clinically meaningful pain response of 10 mm versus 8 (57%) controls. The pain catastrophizing and self-efficacy to manage pain decreased in both groups.
Conclusion: We found that a randomized trial of immersive VR versus a non-immersive control in adolescents with AMPS is feasible and targeted recruitment rates can be achieved. The immersive VR intervention was found to be safe and well-accepted. Most participants and parents expressed willingness to participate in multiple sessions in the future. These findings warrant a future well-powered randomized controlled trial to test the efficacy of this intervention.
To cite this abstract in AMA style:
Joshi S, Zhang Y, Price L, Davis T, Bannuru R. Immersive Virtual Reality for Management of Amplified Musculoskeletal Pain Syndrome in Adolescents: A Pilot Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/immersive-virtual-reality-for-management-of-amplified-musculoskeletal-pain-syndrome-in-adolescents-a-pilot-randomized-controlled-trial/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immersive-virtual-reality-for-management-of-amplified-musculoskeletal-pain-syndrome-in-adolescents-a-pilot-randomized-controlled-trial/