Session Information
Date: Wednesday, November 13, 2019
Title: 6W021: RA – Treatments V: Switching & Tapering RA Medications (2906–2911)
Session Type: ACR Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: The goal of RA treatment is to achieve clinical remission or, at minimum, low disease activity (LDA). Modification of initial csDMARD therapy with the addition of a biologic (b) DMARD, such as a tumor necrosis factor inhibitor (TNFi), or Janus Kinase inhibitor (JAKi) may be needed following an inadequate response (IR).1 Switches in mechanism of action have been advocated in TNF- and JAK-IR patients (pts); however, controlled data describing switches between JAKi and TNFi are lacking.
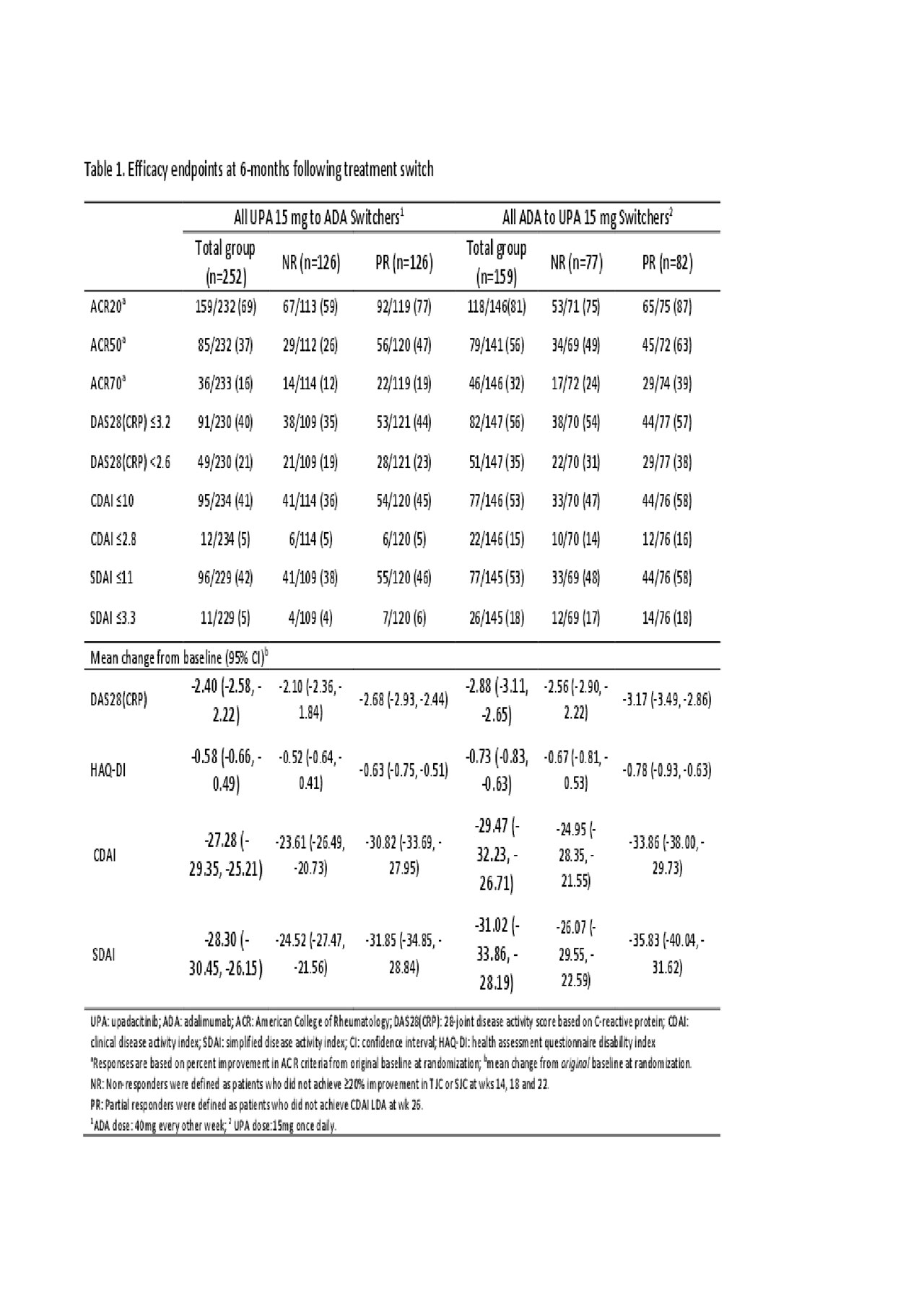
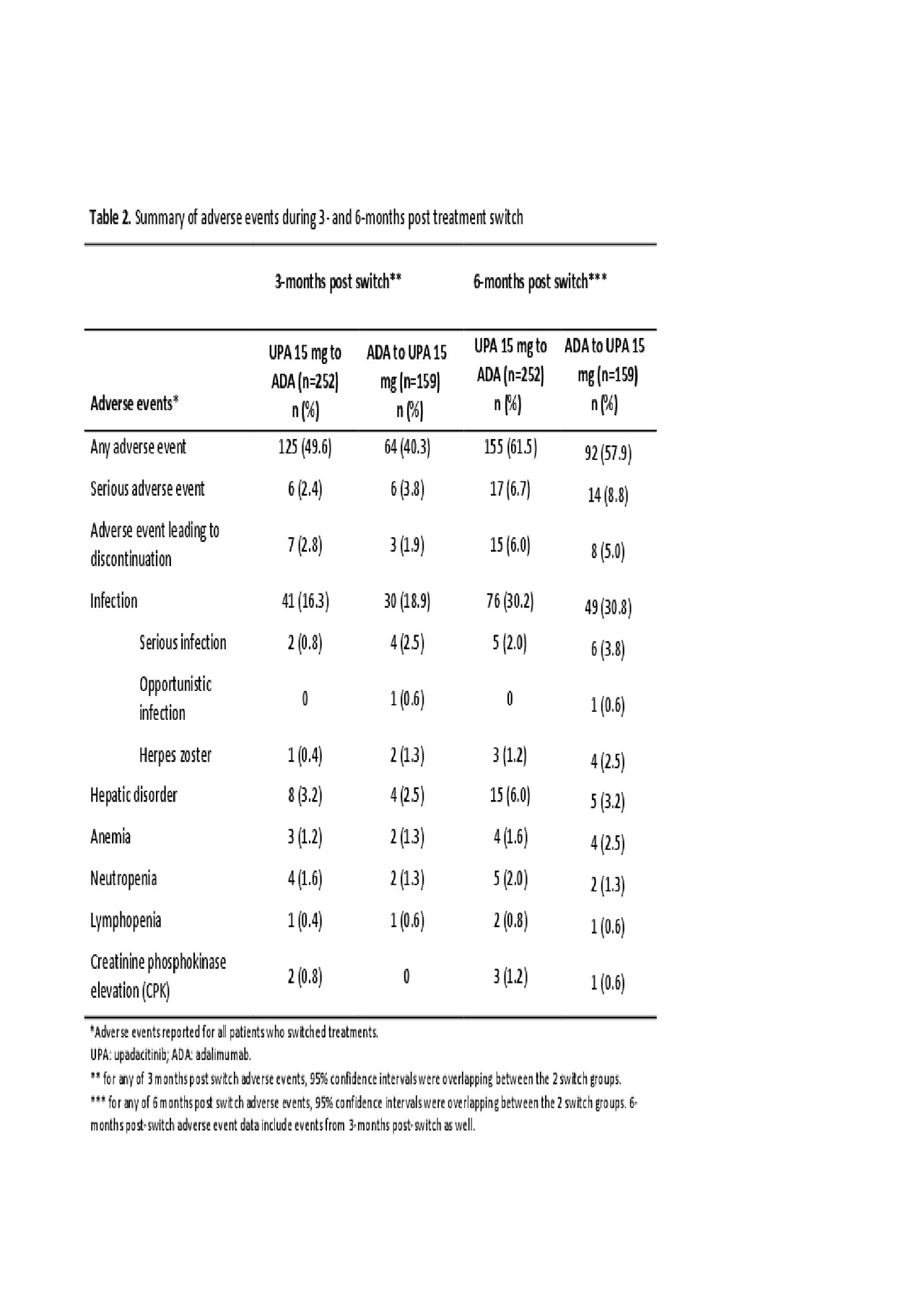
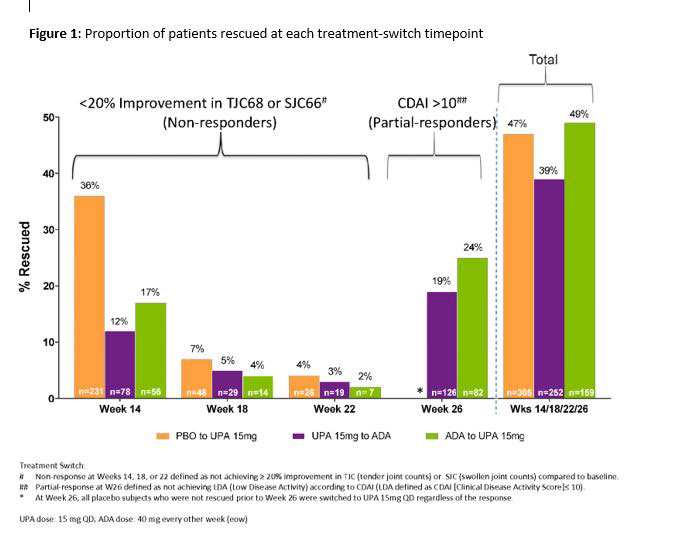
Methods: In a phase 3, double-blind study of the JAK1-selective inhibitor upadacitinib (UPA) 15 mg once daily vs PBO or adalimumab (ADA) 40 mg every other week in MTX-IR pts (all on background MTX), pts without ≥20% improvements from baseline (BL) in tender or swollen joint counts by wks 14, 18, or 22 [non-responders (NR)] were switched from UPA to ADA and vice versa in a blinded fashion and without washout. Pts who did not achieve LDA according to CDAI (CDAI ≤10) at wk 26 [partial-responders (PR)] were switched to the alternate treatment as above. Post-hoc analyses assessed clinical outcomes (Table 1) at 3m(onth) and 6m (±2 wks) post-switch. Adverse events (AEs) were summarized as n% through 3m and 6m post-switch. Data were as observed.
Results: In total, 651 and 327 pts were randomized to receive UPA and ADA. 252 pts were switched from UPA to ADA (39%, NR:126; PR:126), and 159 were switched from ADA to UPA (49%, NR:77; PR:82) (Figure). At 6m post-switch, DAS28(CRP) < 2.6/CDAI ≤2.8 were achieved by 21%/5% of UPA to ADA pts, and 40%/41% achieved DAS28(CRP) ≤3.2/CDAI ≤10. In pts who switched from ADA to UPA, 35%/15% achieved DAS28(CRP) < 2.6/CDAI ≤2.8 and 56%/53% achieved DAS28(CRP) ≤3.2/CDAI ≤10 at 6m post-switch. ACR20/50/70 (based on original BL) after switch are shown in Table 1. Mean change from the original BL in HAQ-DI were -0.58 and -0.73 for pts switching from UPA to ADA and ADA to UPA, respectively. The safety profiles of all pts who switched treatments appeared consistent with those observed for ADA and UPA during comparable periods. During 3m post-switch, the proportions (n%) of pts with serious AEs were 2.4% and 3.8% in the UPA-ADA and ADA-UPA groups, respectively; while serious infections were reported in 0.8% in the UPA-ADA group and in 2.5% in the ADA-UPA group. Safety at 6m overall was comparable to 3m post-switch (Table 2).
Conclusion: Data from this blinded, controlled study indicate that pts with initial insufficient response, either non-response or partial response, to either UPA or ADA may benefit from switching to the other therapy. No additional safety concerns were observed. These data suggest that a patient failing either UPA or ADA may respond to the alternate therapy.
References:
- Singh, et al. Arthritis Rheumatol, 2016;68:1-26.
To cite this abstract in AMA style:
Fleischmann R, Genovese M, Blanco R, Hall S, Thomson G, Van den Bosch F, Zerbini C, Enejosa J, Li Y, DeMasi R, Song I. Clinical and Functional Outcomes Among Rheumatoid Arthritis Patients Switching Between JAK1-Selective Inhibitor Upadacitinib and Adalimumab Following Insufficient Response [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinical-and-functional-outcomes-among-rheumatoid-arthritis-patients-switching-between-jak1-selective-inhibitor-upadacitinib-and-adalimumab-following-insufficient-response/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-and-functional-outcomes-among-rheumatoid-arthritis-patients-switching-between-jak1-selective-inhibitor-upadacitinib-and-adalimumab-following-insufficient-response/