Session Information
Date: Wednesday, November 13, 2019
Title: 6W020: Miscellaneous Rheumatic & Inflammatory Disease III: Novel Therapies (2900–2905)
Session Type: ACR Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Interstitial Lung Disease (ILD) is the most common lung manifestation in Connective Tissue Diseases (CTD). It is present in most types of CTD, such as: Rheumatoid Arthritis (RA), Systemic Sclerosis (SSc), Systemic Lupus Erythematous (SLE), Inflammatory Myositis (IM), Mixed Connective Tissue Disease (MCTD), Sjogren Syndrome and Sarcoid. Despite it being so common, treatment has lacked to advance in the biologic era of Rheumatology. CTD-ILD has different histological patterns, the most common being non-specific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), and organizing pneumonia (OP). Literature has showed that Rituximab (RTX), a B lymphocyte depleting monoclonal antibody, can be useful in CTD-ILD. For that reason, we decided to evaluate the use and safety of RTX in patients who failed conventional immunosuppressant therapy.
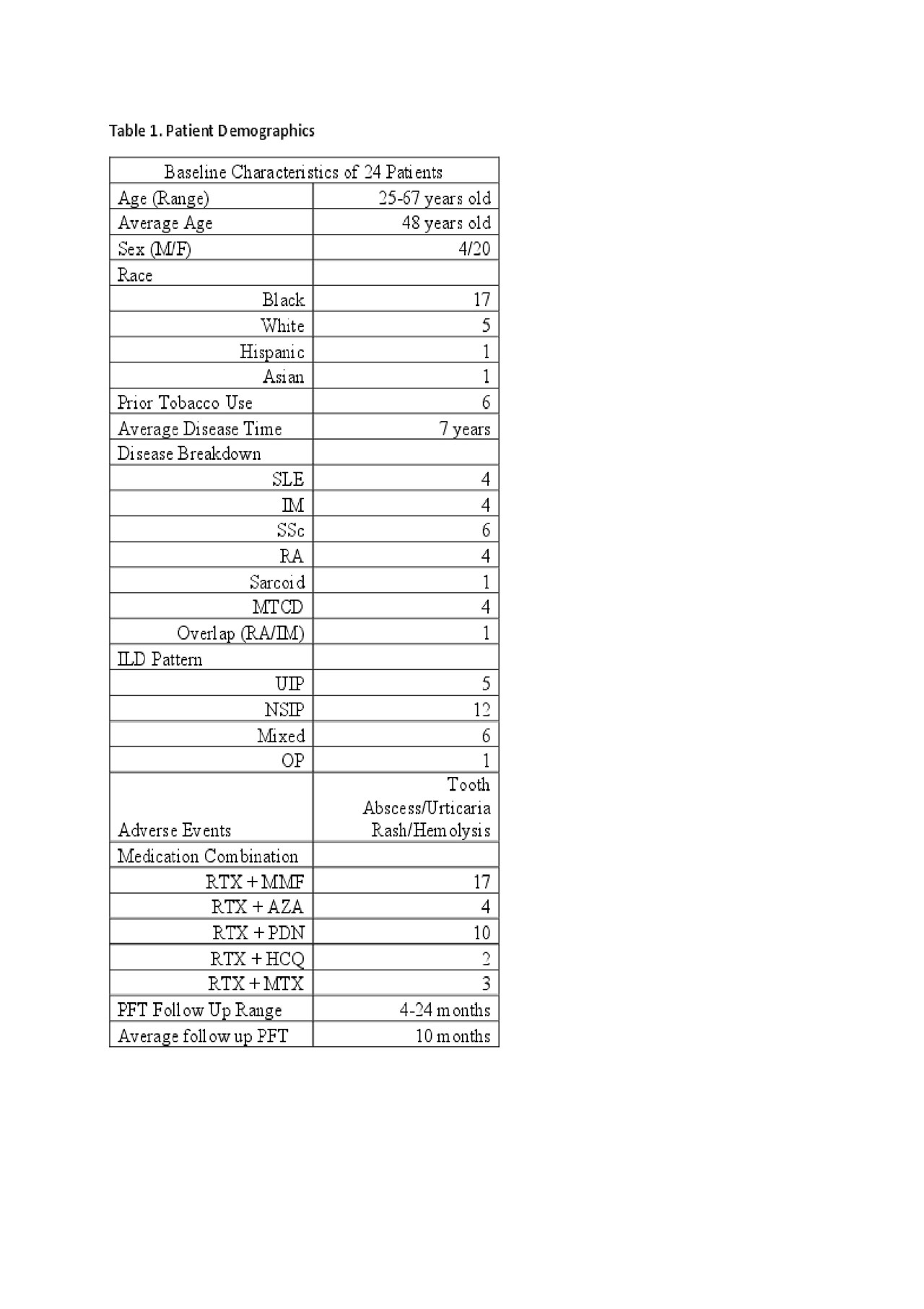
Methods: In a retrospective chart review, we identified all patients with CTD-ILD expanding from 2015 to 2018, seen in the Rheumatology Clinic of University Medical Center New Orleans, Louisiana (UMCNO). This was done by performing a review of our clinical database by cross referencing International Classification of Diseases (ICD) 10 code J84.9 & 84.10, with M35.9, M05.10, M34.81, D89.89, M32.1, G72.4, M35.02, M35.0. Eighty charts were retrospectively reviewed. Total of 24 patients were given RTX in addition to other immunosuppressive therapy. Data extracted from the chart included patient age, sex, race, tobacco history, CTD history, ILD histological pattern based on High Resolution Computer Tomography (HRCT) of the chest, pre and post pulmonary function test (PFT), other therapy used, and side effects after RTX.
Results: The patients average age was 48 years old. The study had a female to male ratio of 5:1, with 17 of 24 being of African descent. Of the 24, six had prior tobacco use. The most common CTD in our cohort was SSc with 6 of 24, then SLE, IM, RA and MCTD. There was one case of Sarcoid and another of an overlap of RA/IM. Average disease onset at time of RTX infusion was 7 years. The average PFT post RTX therapy was 10 months. NSIP was the most common ILD pattern seen in 12 of 24, then mixed pattern 6 of 24, UIP 5 of 24, and one OP. RTX was most commonly combined with Mycophenolate, 17 of 24 patients were receiving both therapies. Low dose prednisone (< 7.5mg/daily) was concurrently being given in 10 of 24 patients. Only 3 adverse events were encountered during chart review post RTX infusion: Tooth Abscess, Urticarial Rash, and Hemolysis. Pre and Post RTX PFTs are in the table below showing in average stability of the values.
Conclusion: As our data shows RTX is not only safe in CTD-ILD but also proves that it provides stability in lung function on repeat PFT at 10 months average. Our cohort was unique in that the majority were African Americans, and that it included a wide array of ILD patterns and CTD. We also demonstrated that it can be combined with other immunosuppressants and the side effect profile is not much different than using it by itself. As well as no clear trend towards improvement with any combination of immunosuppressants.
To cite this abstract in AMA style:
Mesa C, Yadlapati S, Guevara M. The Use and Safety of Rituximab in Connective Tissue Disease Associated Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-use-and-safety-of-rituximab-in-connective-tissue-disease-associated-interstitial-lung-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-use-and-safety-of-rituximab-in-connective-tissue-disease-associated-interstitial-lung-disease/