Session Information
Session Type: ACR Abstract Session
Session Time: 9:00AM-10:30AM
Background/Purpose: The ultimate goal of therapy in patients with psoriatic arthritis (PsA) is clinical remission, defined as ‘the absence of clinical and laboratory evidence of significant inflammatory disease activity’.1,2 Since many patients with PsA may not achieve clinical remission, treatment recommendations recognise minimal or low disease activity as an important alternative treatment target.1,2 Bimekizumab is in development for the treatment of psoriasis, PsA, and axial spondyloarthritis; it potently and selectively neutralizes both IL-17A and IL-17F.3 The efficacy and safety of bimekizumab in patients with active PsA was assessed in a Phase 2b study over 48 weeks (NCT02969525);4 disease activity outcomes are reported here.
Methods: Patients with active PsA, ≥3/76 swollen joint count and ≥3/78 tender joint count and fulfilling the Classification Criteria for PsA (CASPAR, score ≥3), were randomized (1:1:1:1:1) to receive subcutaneous bimekizumab 16 mg, 160 mg, 160 mg with 320 mg loading dose (160 mg [LD]), 320 mg, or placebo every 4 weeks (Q4W) for 12 weeks (double-blind period). After Week 12, patients receiving placebo or bimekizumab 16 mg were re-randomized (1:1) to receive bimekizumab 160 mg or 320 mg; all other patients continued on their previous dose (dose-blind period). The primary endpoint was a ≥50% improvement in American College of Rheumatology response criteria (ACR50) at Week 12; key results up to Week 48 have been reported previously.4 Other and post-hoc efficacy variables included: minimal disease activity (MDA), very low disease activity (VLDA), disease activity index for psoriatic arthritis (DAPSA) remission, and DAPSA low disease activity (LDA).
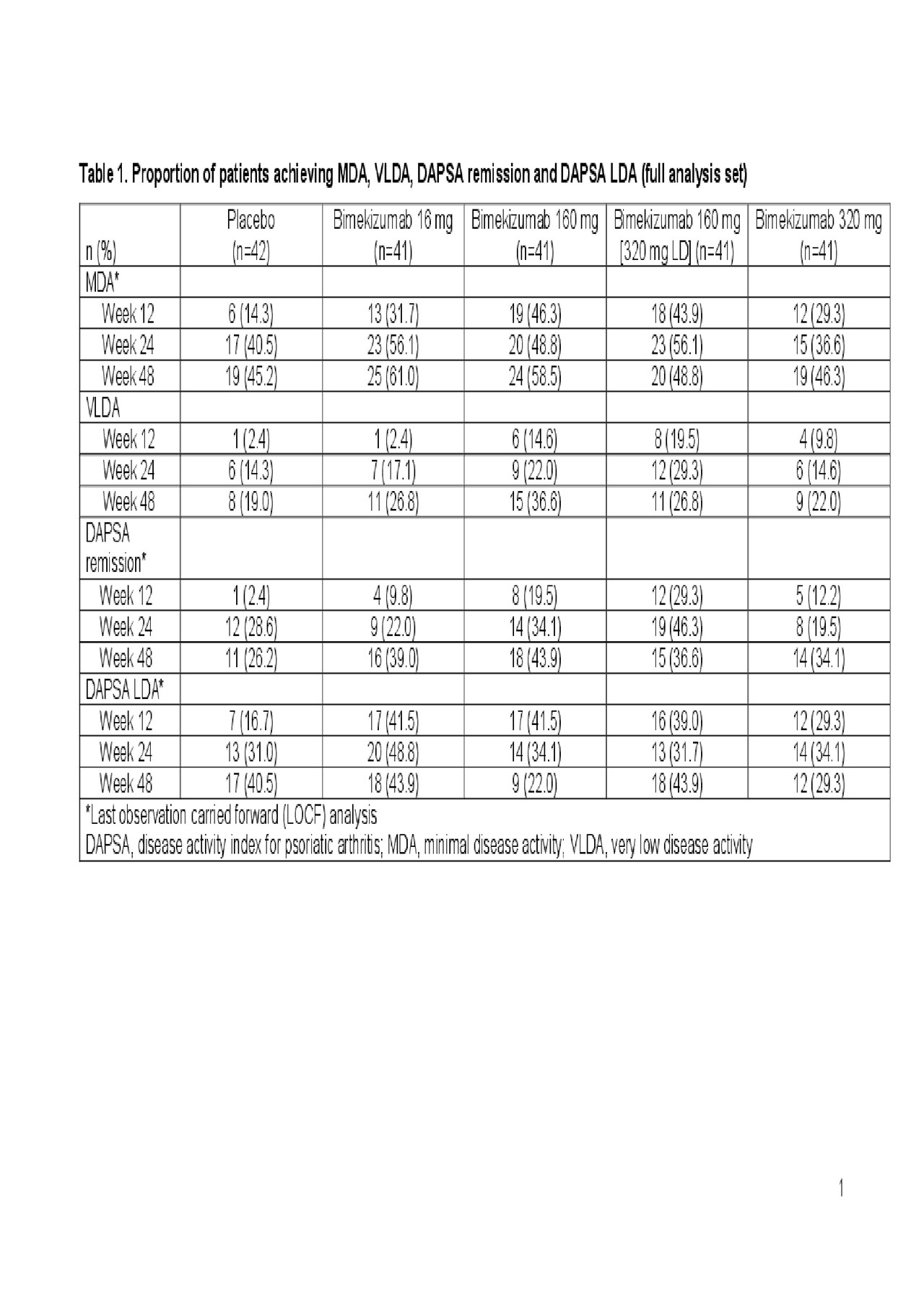
Results: Of 206 patients randomized, 203 and 190 completed the double- and dose-blind periods, respectively. In patients receiving bimekizumab 160 mg, 160 mg (LD) or 320 mg throughout the study, 46.3–58.5% achieved MDA, 22.0–36.6% achieved VLDA, 34.1–43.9% achieved DAPSA remission and 22.0–43.9% achieved DAPSA LDA at Week 48 (Table 1). In general, disease activity improved across these measures from Week 12 to Week 24, with improvements typically maintained or further increased to Week 48.
Serious adverse events were reported by 9/204 (4.4%) patients up to Week 48 (8/204 [3.9%] receiving bimekizumab). The most common treatment-emergent adverse event up to Week 48 was nasopharyngitis (25/204 [12.1%]). Up to Week 48, oral candidiasis occurred in 10/204 (4.9%) patients (all on bimekizumab treatment). No deaths, cases of inflammatory bowel disease, uveitis, or major cardiovascular adverse events were reported.
Conclusion: Treatment with bimekizumab 160 mg, 160 mg (LD) or 320 mg throughout the study was associated with achievement of low and/or minimal disease activity. Disease activity measures generally improved to Week 24 and were maintained to Week 48. These data provide further support that neutralizing both IL‑17F and IL-17A with bimekizumab is a promising therapeutic approach in patients with active PsA.
References: 1Smolen Ann Rheum Dis 2018;77:3–17; 2Gossec Ann Rheum Dis 2016;75:499–510; 3Papp JAAD 2018;79:277–86; 4Ritchlin Arthritis Rheumatol 2018;70(suppl 10):L17.
Mease_PA0008 ACR abstract_Final_03Jun19_Table
To cite this abstract in AMA style:
Mease P, Gossec L, Coates L, Gottlieb A, Assudani D, Ink B, Coarse J, Irvin-Sellers O, Gladman D. Dual Neutralization of IL-17A and IL-17F with Bimekizumab in Patients with Active Psoriatic Arthritis: Disease Activity and Remission in a 48-Week Phase 2b, Randomized, Double‑Blind, Placebo-Controlled, Dose-Ranging Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/dual-neutralization-of-il-17a-and-il-17f-with-bimekizumab-in-patients-with-active-psoriatic-arthritis-disease-activity-and-remission-in-a-48-week-phase-2b-randomized-double%e2%80%91blind-placebo-c/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/dual-neutralization-of-il-17a-and-il-17f-with-bimekizumab-in-patients-with-active-psoriatic-arthritis-disease-activity-and-remission-in-a-48-week-phase-2b-randomized-double%e2%80%91blind-placebo-c/
