Session Information
Session Type: Plenary Session III
Session Time: 11:00AM-12:30PM
Background/Purpose: Osteoporosis and renal insufficiency are coexisting disease states in a substantial proportion of postmenopausal women. Since bisphosphonates are generally contraindicated in patients with estimated GFR (eGFR) < 35 mL/min, it is important to evaluate other osteoporosis treatments in this setting. This post hoc analysis of the FRAME study assessed the efficacy and safety of romosozumab (Romo) vs placebo (Pbo) among patients with different levels of renal function.
Methods: FRAME enrolled 7,180 postmenopausal women with T-scores –2.5 to –3.5 at the total hip (TH) or femoral neck (FN). In the Pbo-controlled double-blind treatment phase, patients received Romo 210 mg or Pbo monthly for 12 months. Patients were grouped by baseline eGFR (mL/min/1.73m2) normalized to body surface area, calculated using the MDRD study equation. Renal function was categorized as normal renal function (eGFR ≥ 90) or mild (eGFR 60–89 [chronic kidney disease (CKD) stage 2]), moderate (eGFR 30–59 [CKD stage 3]), or severe (eGFR 15–29 [CKD stage 4]) renal insufficiency. The least squares mean % change from baseline in bone mineral density (BMD) at the lumbar spine (LS), TH, and FN; incidence of new vertebral fractures and adverse events; and CKD progression were assessed for each eGFR category at month 12.
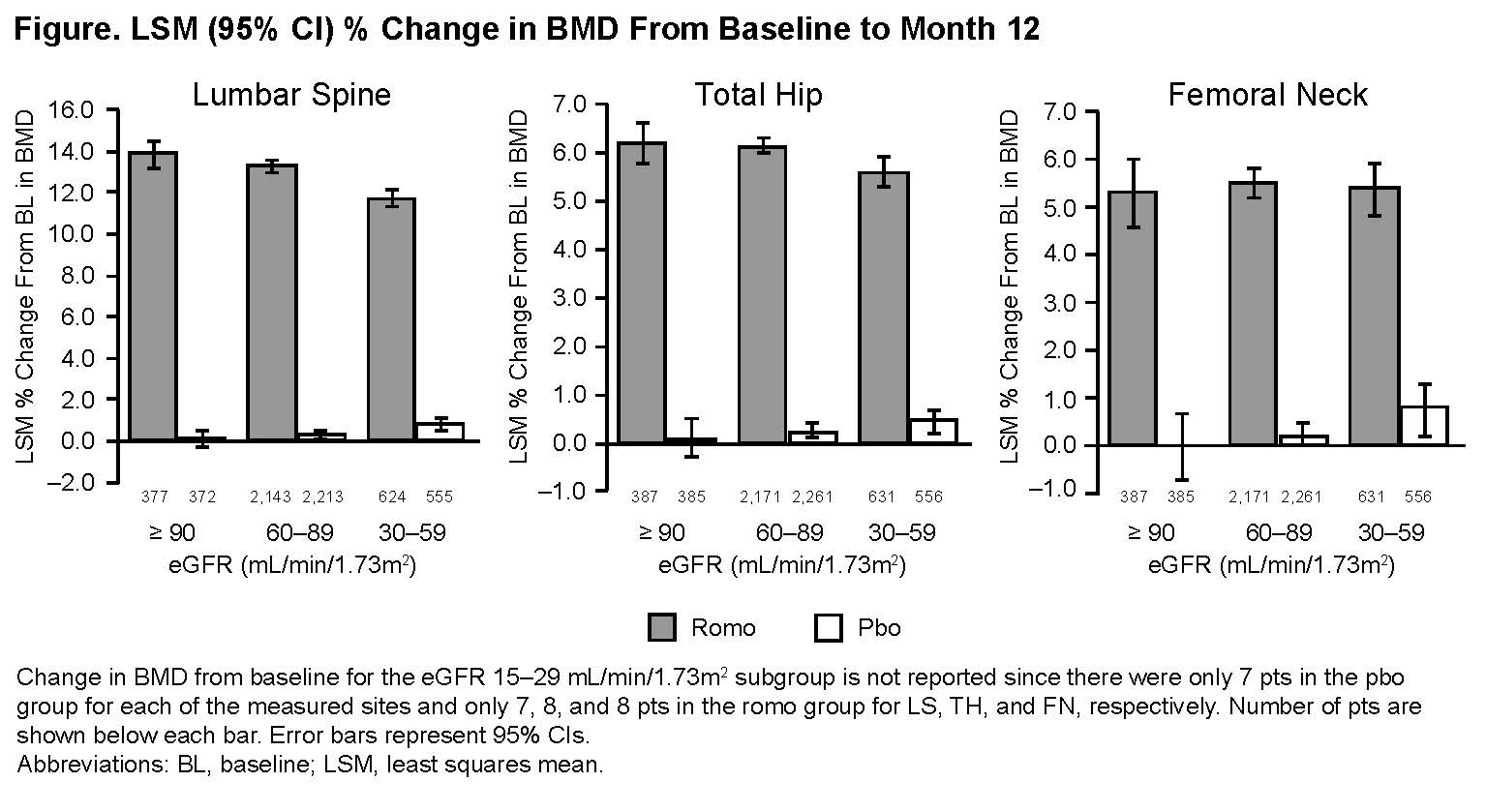
Results: At baseline, most patients (88%) had mild or moderate renal insufficiency; 0.3% had severe renal insufficiency. In the overall patient population, the least squares mean % change (95% CI) from baseline in BMD (Romo vs Pbo) was 13.1% (12.8–13.3) vs 0.4% (0.2–0.5) for LS, 6.0% (5.9–6.2) vs 0.3% (0.1–0.4) for TH, and 5.5% (5.2–5.7) vs 0.3% (0.1–0.5) for FN. Changes in BMD were similar irrespective of baseline eGFR (Figure). Incidence of new vertebral fractures (Romo/Pbo) in patients with normal renal function, CKD 2, or CKD 3 was 0.5%/3.0%, 0.4%/1.5%, and 0.6%/2.1%, respectively. The overall incidence of adverse events and serious adverse events, and the incidence of positively adjudicated cardiovascular events were similar between treatment groups for all eGFR categories. One patient (CKD 2 at baseline) in the Romo group had grade 2 hypocalcemia. Mild-to-moderate calcium decrease occurred in 4 patients in the Pbo group and 13 patients in the Romo group. Most patients (approximately 80% in each group) had no changes from baseline eGFR category at the end of the 12-month period.
Conclusion: Romo increased BMD across eGFR subgroups vs Pbo, and the reduction in new vertebral fractures was not notably affected by eGFR level. Safety was also generally comparable among eGFR subgroups.
To cite this abstract in AMA style:
Miller P, Chines A, Albergaria B, Gielen E, Langdahl B, Miyauchi A, Vanderkelen M, Milmont C, Maddox J, Adachi J. Efficacy and Safety of Romosozumab vs Placebo Among Patients with Mild-to-Moderate Chronic Kidney Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-romosozumab-vs-placebo-among-patients-with-mild-to-moderate-chronic-kidney-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-romosozumab-vs-placebo-among-patients-with-mild-to-moderate-chronic-kidney-disease/