Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Prior to the development of anti-cytokine therapies, treatment for systemic juvenile idiopathic arthritis (SJIA) included high dose glucocorticoids and non-biologic disease modifying anti-rheumatic drugs (DMARDs) with suboptimal outcomes. Recent studies of SJIA patients treated with biologics, including IL-1 inhibitors (IL-1i) and IL-6 inhibitors (IL-6i), demonstrated excellent clinical responses, with steroid-sparing benefits and improved short-term outcomes. Our aim was to assess temporal trends in non-biologic DMARD and biologic medication use in the first year after diagnosis of SJIA using data from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry.
Methods: Medication use during the first year after diagnosis of SJIA was assessed for all patients enrolled in the CARRA Registry through 2017. Medication information is collected retrospectively for all patients at the time of enrollment in the Registry and prospectively after enrollment. Patients with missing diagnosis date or month of medication start date were excluded. Patients were grouped by year of diagnosis based on medication availability at the time and numbers of patients available for assessment: (1)1998-2004: little biologic use; (2) 2005-2010: mostly anakinra use; (3) 2011-2013: post-FDA approval of tocilizumab and canakinumab; (4) 2014-2015; and (5) 2016-2017. Medications were grouped by mechanism of action, and temporal trends in medication class usage were assessed using frequencies.
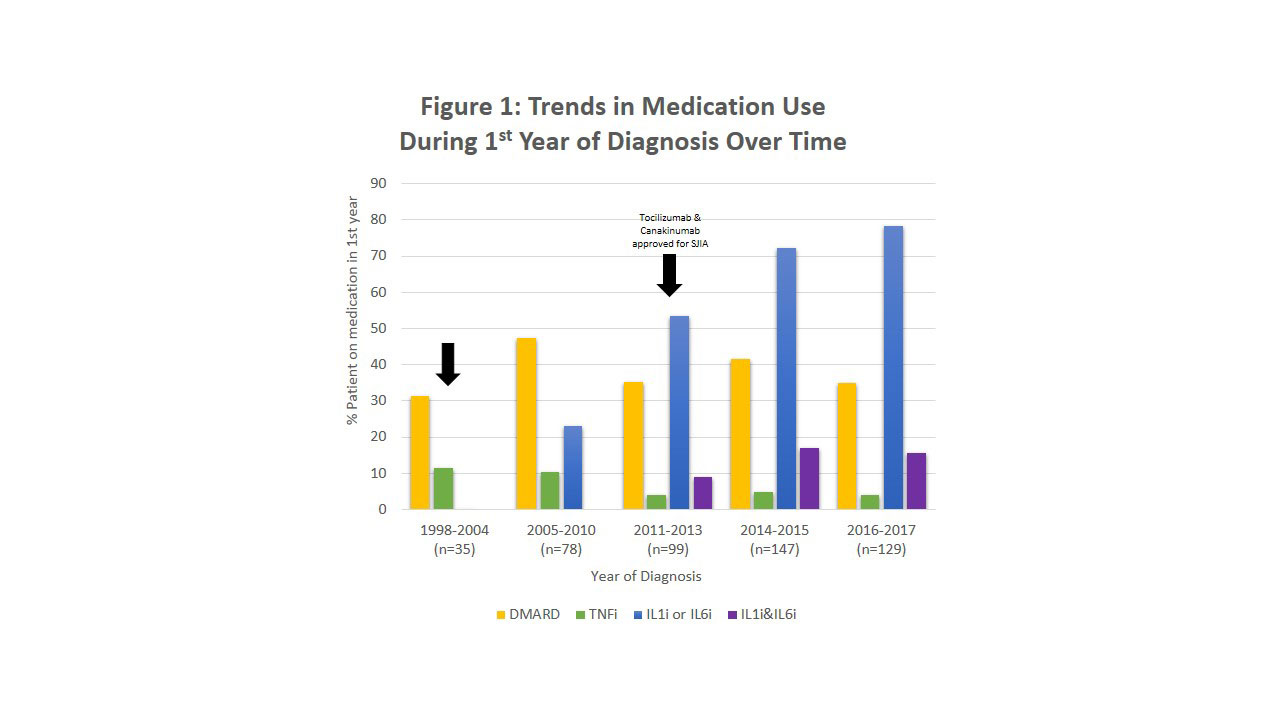
Results: A total of 488 patients were included in the analysis: 35 patients in the 1998-2004 cohort, 78 from 2005-2010, 99 from 2011-2013, 147 from 2014-2015, and 129 from 2016-2017 (Figure 1). Non-biologic DMARD use fluctuated between 31% and 47% over the study period. Use of IL-1i or IL-6i consistently increased over time to >75% by 2017. In recent years, approximately 15% of patients received both IL-1i and IL-6i, and TNF inhibitor (TNFi) use decreased to < 5% in the first year.
Conclusion: Our analysis shows a dramatic increase over time in use of IL-1i and IL-6i in the first year of treatment for SJIA patients, with over 75% of patients now receiving these treatments. Use of non-biologic DMARDs was stable over the study period, indicating a change in the standard of care. Use of both IL-1i and IL-6i by a significant minority of patients suggests variability of clinical response in individual patients.
To cite this abstract in AMA style:
Janow G, Beukelman T, Kimura Y, Schneider R, Mohan S, Rodich G, Son M. Increasing Use of Biologics over Time in the First Year After Diagnosis of Systemic JIA Among Patients Enrolled in the Childhood Arthritis & Rheumatology Research Alliance (CARRA) Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/increasing-use-of-biologics-over-time-in-the-first-year-after-diagnosis-of-systemic-jia-among-patients-enrolled-in-the-childhood-arthritis-rheumatology-research-alliance-carra-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/increasing-use-of-biologics-over-time-in-the-first-year-after-diagnosis-of-systemic-jia-among-patients-enrolled-in-the-childhood-arthritis-rheumatology-research-alliance-carra-registry/