Session Information
Date: Tuesday, November 12, 2019
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III: Giant Cell Arteritis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Giant Cell Arteritis (GCA) is a type of large vessel vasculitis that can cause blindness and aortic aneurysms. A significant unmet medical need remains in GCA, as current treatment options are limited and relapse increases corticosteroid (CS) exposure and toxicity.
The primary role of macrophages/dendritic cells (DCs) and TH1/TH17 lymphocytes in GCA pathogenesis has been highlighted previously. Granulocyte-macrophage colony stimulating factor (GM-CSF) may contribute to GCA pathogenesis by stimulating giant cell formation. GM-CSF produced by CD4+ T helper TH1 and TH17 cells can stimulate conventional DCs and promote differentiation of monocyte-derived DCs. Notably GM-CSF RNA has been reported in GCA lesions and in peripheral blood mononuclear cells of symptomatic patients.
We hypothesized elevation of the GM-CSF pathway signature in GCA vessels versus controls.
Methods: Two independent sources of temporal artery biopsies were utilized. First, GCA (n=17) and control (symptomatic patients suspected for GCA, but with a normal temporary artery biopsy; n=5) biopsies were analyzed for 15 mRNA transcripts representing TH1, TH17, and GM-CSF signaling (RNAscope; RS) and for mRNA transcripts representing the autoimmune panel (Nanostring; NS). Semi-quantitative scoring was performed on RS images and fold-change of representative TH1, TH17 and GM-CSF related mRNA transcripts were calculated via NS nCounter analysis. Additional GCA and control biopsies were obtained and analyzed by qPCR for a subset of transcripts (n=10 each) and by confocal microscopy for GM-CSF and GM-CSF-Rα protein (n=2 each). Ex vivo cultures of GCA+ arteries were treated with placebo or mavrilimumab for 5 days and assayed for gene expression by qPCR.
Results: Upregulation of the GM-CSF signaling pathway molecular signature was confirmed by 4 independent analyses.
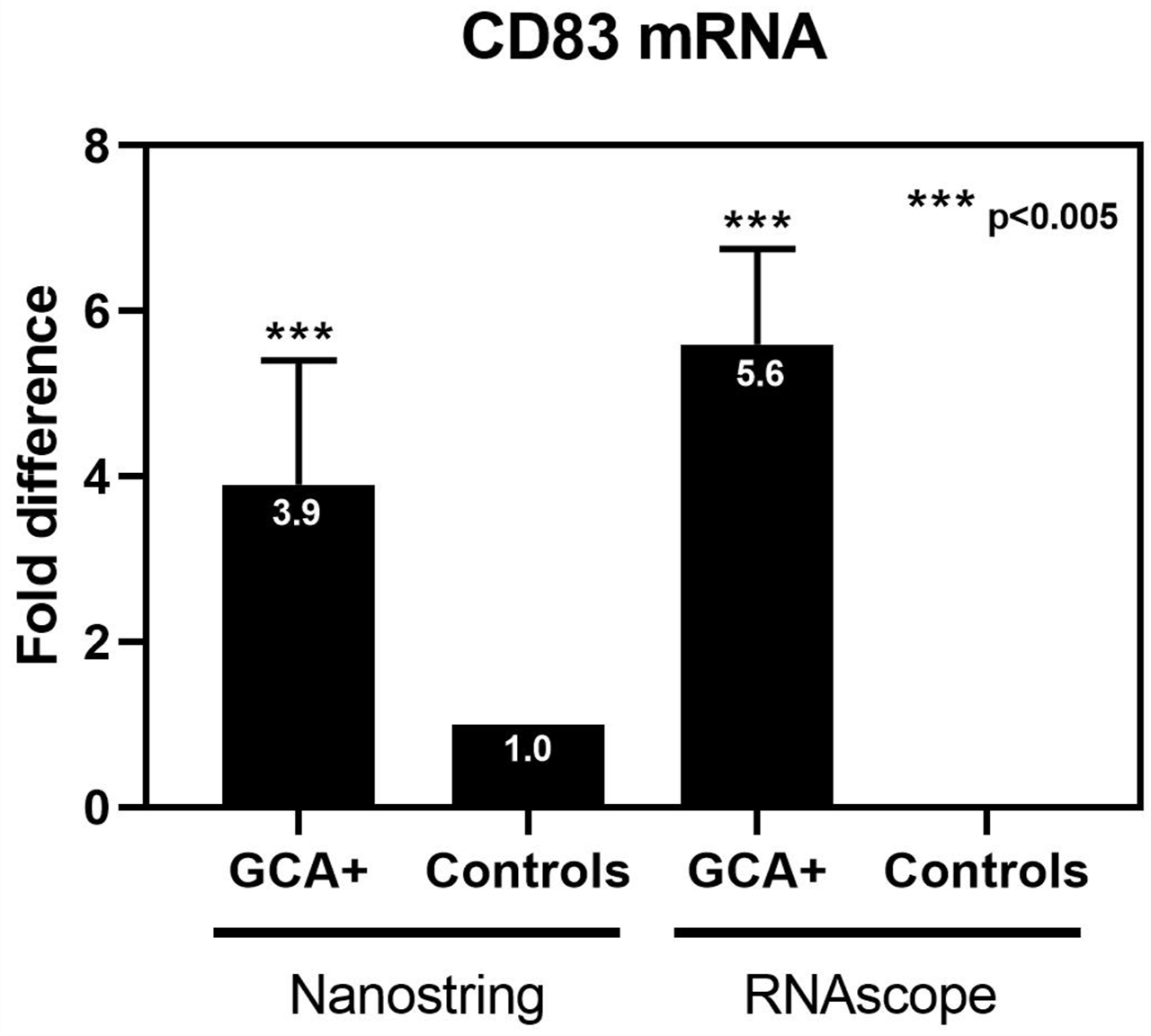
GM-CSF-associated and TH1-associated genes were upregulated in GCA biopsies versus control (GMCSF: 3-4x RS; GM-CSF-Rα: 6.7x NS, 6x RS; and CD83: 3.9x NS, 6x RS; TNFα: 2x NS, 3x RS; IFNγ: 2x RS; IL-1β: 6x RS). TH17 associated genes were not elevated, potentially due to concomitant CS treatment.
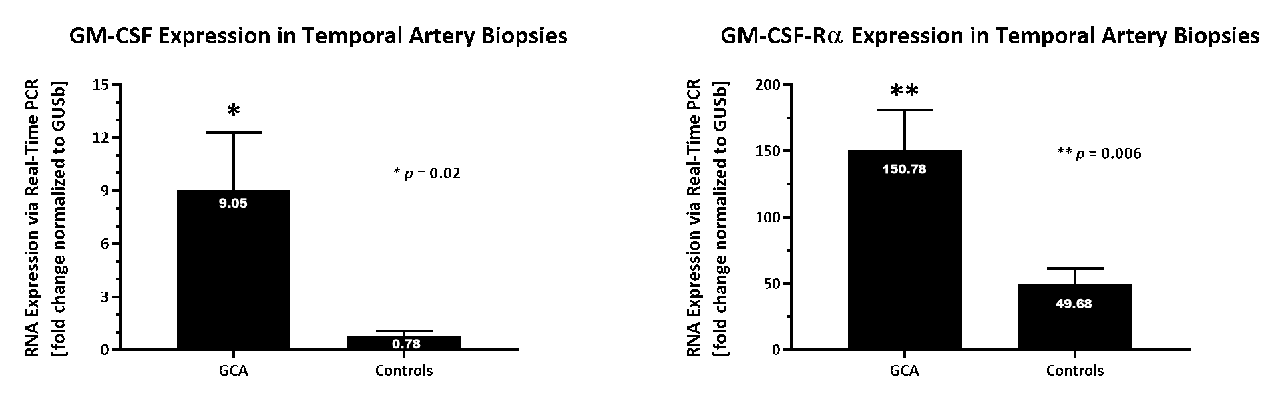
Upregulation of both GM-CSF (12x) and GM-CSF-Rα (3x) mRNA was confirmed in a separate cohort of biopsies from GCA pts vs. controls by qPCR (Figure). GM-CSF protein was detected in the luminal endothelium, neovessels and inflammatory cells of GCA patients. In normal temporal arteries GM-CSF protein was not detected.
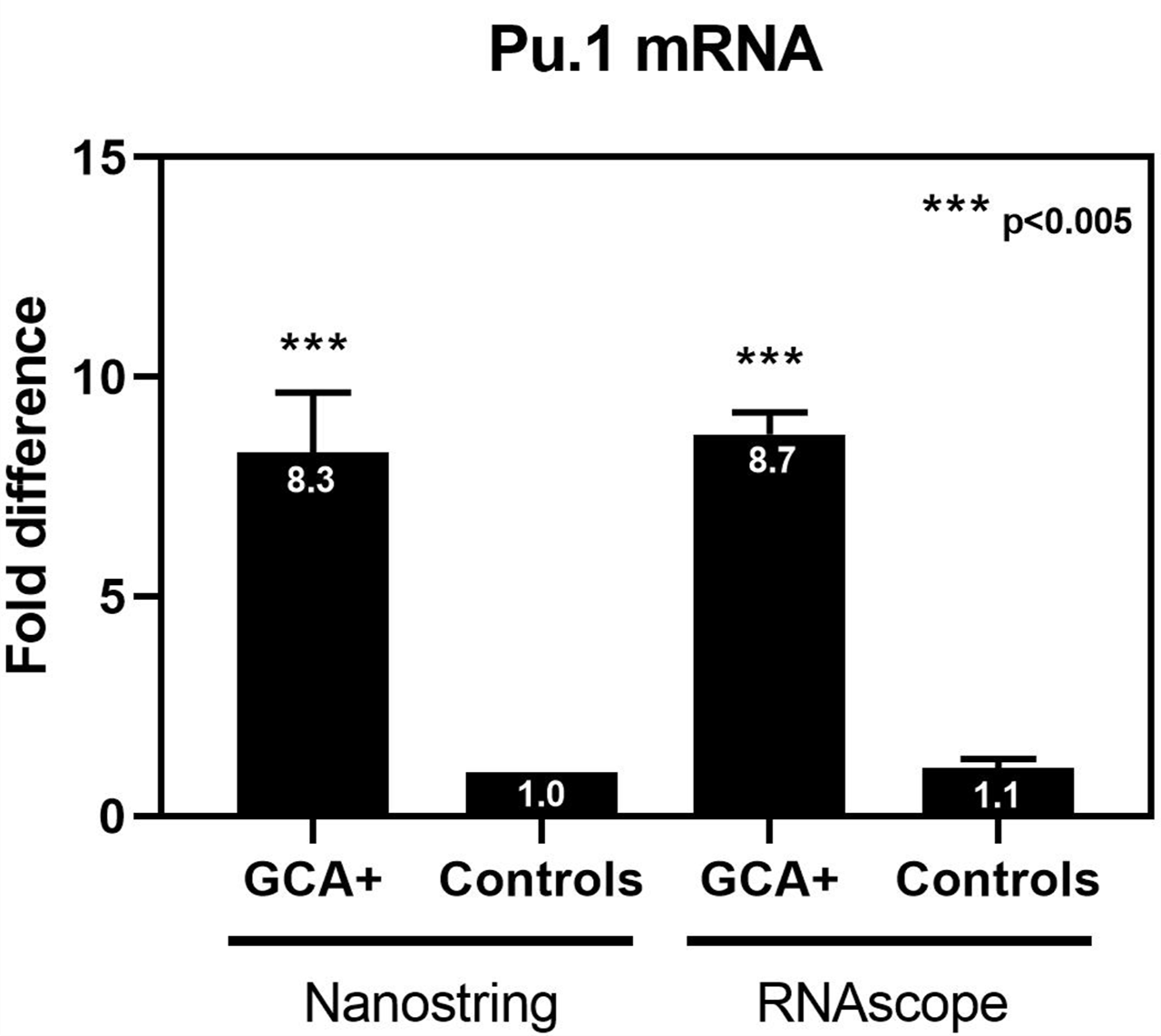
Pu.1, a transcription factor downstream of GM-CSF signaling, was increased 8x in GCA vs. controls (RS, NS) (Figure)
Treatment of ex vivo cultures of GCA arteries with Mavrilimumab (anti-GM-CSF-Rα) reduced expression patterns of known pathogenic cell-types and cytokines (CD3ε, CD83, HLA-DRA Pu.1, and TNFα).
Conclusion: GM-CSF and TH1 pathway signatures were demonstrated in GCA patient temporal arteries by independent analytical techniques. Active GM-CSF signaling in diseased tissue is evidenced by increased expression of Pu.1 in the vessel wall. Treatment with mavrilimumab reduced inflammatory cell markers and pathogenic cytokines. These data implicate the GM-CSF pathway in GCA pathophysiology and increase confidence in rationale for targeting GM-CSF in GCA.
To cite this abstract in AMA style:
Cid M, Gandhi R, Corbera-Bellalta M, Terrades-Garcia N, Muralidharan S, Paolini J. GM-CSF Pathway Signature Identified in Temporal Artery Biopsies of Patients with Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/gm-csf-pathway-signature-identified-in-temporal-artery-biopsies-of-patients-with-giant-cell-arteritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gm-csf-pathway-signature-identified-in-temporal-artery-biopsies-of-patients-with-giant-cell-arteritis/