Session Information
Date: Tuesday, November 12, 2019
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III: Giant Cell Arteritis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tocilizumab (TCZ) administered subcutaneously weekly or every 2 weeks with a 26-week prednisone taper (TCZ-QW or TCZ-Q2W) was superior to placebo given with a 26-week or 52-week prednisone taper for the achievement of sustained glucocorticoid (GC)-free remission in patients with giant cell arteritis (GCA) in part 1 of the double-blind phase 3 GiACTA trial.1 Part 2 was a 2-year, open-label, long-term extension in which TCZ could be stopped or started as needed to achieve disease control. In this analysis, the impact of TCZ dosing interruption was assessed on pharmacokinetic (PK) and pharmacodynamic (PD) end points.
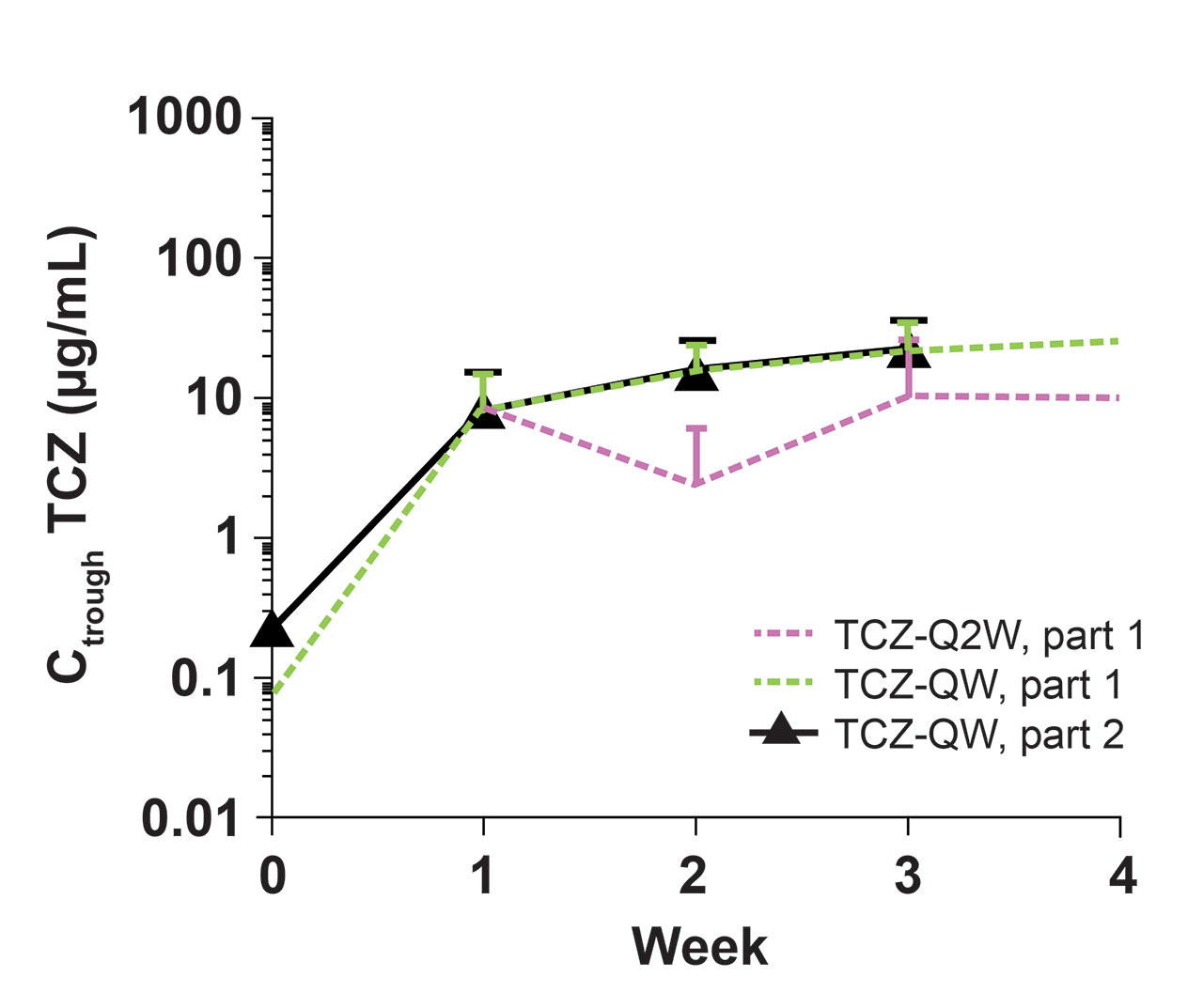
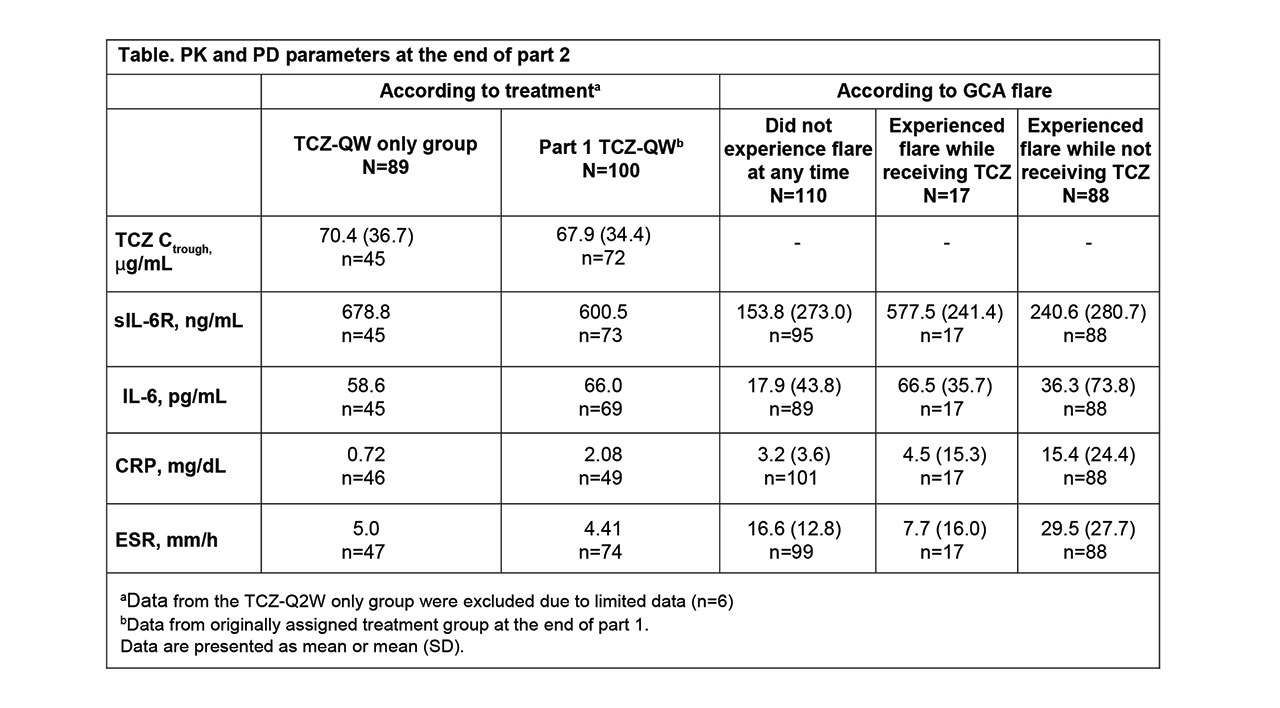
Methods: PK was assessed as predose serum TCZ concentrations (Ctrough), and serum PD biomarkers measured were interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R), C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). To assess the impact of TCZ on PK/PD during initial drug exposure and at steady state, data were assessed in patients receiving their first 4 doses in part 2 after a dosing interruption of ≥4 weeks (TCZ-break group) and in patients who had received TCZ QW ≥12 weeks (TCZ-QW group). The 4-week dose interruption was included to allow drug levels to fall to negligible levels in patients treated with TCZ in part 1, thereby allowing data from patients who received placebo or TCZ in part 1 to be pooled for part 2 analyses. The 12-week dosing period in part 2 was based on the time to reach steady state after TCZ-QW dosing as determined in part 1. Data from part 1 were used for comparison. PD parameters from patients who experienced flare in part 2 were also examined to determine the prognostic value of biomarkers, regardless of treatment assignment.
Results: Among 250 patients who received treatment in part 1 (TCZ-QW, n = 100; TCZ-Q2W, n = 49; PBO+26, n = 50; PBO+52, n = 51), 215 entered part 2. Seventy patients had a ≥4-week dosing interruption, received the first 4 consecutive TCZ-QW doses in part 2, and were included in the TCZ-break group. During the first 4 weeks of dosing, TCZ Ctrough levels were comparable in the TCZ-break group and the part 1 TCZ-QW group (Figure). In part 2, TCZ Ctrough and PD biomarkers after the first initial 4 doses and at steady state were comparable to the values at the end of part 1 (Table). Patients who experienced flare while on TCZ had low levels of CRP and ESR. Patients who experienced flare while not receiving TCZ had higher levels of CRP and ESR compared to those who experienced flare on TCZ, where flares occurred despite low levels of these inflammatory markers.
Conclusion: Restarting TCZ-QW treatment after dose interruption resulted in exposure and PD biomarker profiles similar to those observed with double-blind TCZ-QW treatment, suggesting there were no negative effects for patients with GCA who stopped and restarted TCZ. Low levels of CRP and ESR confirm that despite adequate control by TCZ, these biomarkers are not the sole indicators of flare in GCA patients receiving TCZ, but, in the absence of TCZ, they could serve as indicators of risk for flare.
Reference: 1. Stone JH et al. N Engl J Med 2017;377:317-328.
To cite this abstract in AMA style:
Malallieu N, Bao M, Stone J. Pharmacokinetics and Pharmacodynamics of Tocilizumab in Combination with Prednisone Tapering in Patients with Giant Cell Arteritis: 3-Year Results from a Randomized Controlled Phase 3 Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pharmacokinetics-and-pharmacodynamics-of-tocilizumab-in-combination-with-prednisone-tapering-in-patients-with-giant-cell-arteritis-3-year-results-from-a-randomized-controlled-phase-3-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetics-and-pharmacodynamics-of-tocilizumab-in-combination-with-prednisone-tapering-in-patients-with-giant-cell-arteritis-3-year-results-from-a-randomized-controlled-phase-3-trial/