Session Information
Date: Tuesday, November 12, 2019
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III: Giant Cell Arteritis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
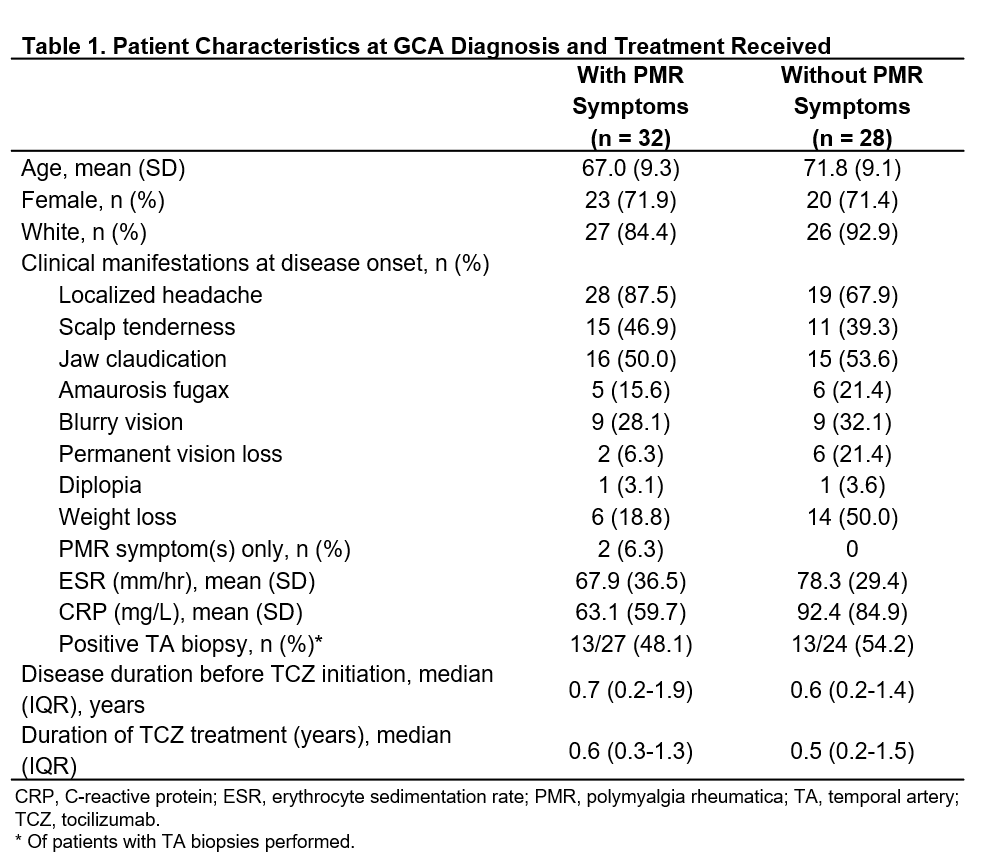
Background/Purpose: Approximately 50% of patients with giant cell arteritis (GCA) also have polymyalgia rheumatica (PMR) symptoms.1 The purpose of this study was to determine whether the presence of PMR symptoms upon GCA diagnosis impacts GCA clinical outcomes and to evaluate the effectiveness of tocilizumab (TCZ) for controlling PMR symptoms in patients with GCA.
Methods: This was a retrospective analysis of patients with GCA treated with TCZ at a single center (2010-2018). Disease flares, defined as re-appearance of clinical manifestations (eg, cranial or PMR signs or symptoms), were assessed among patients with or without PMR at the time of GCA diagnosis. PMR symptoms during disease flares were assessed before and after TCZ initiation.
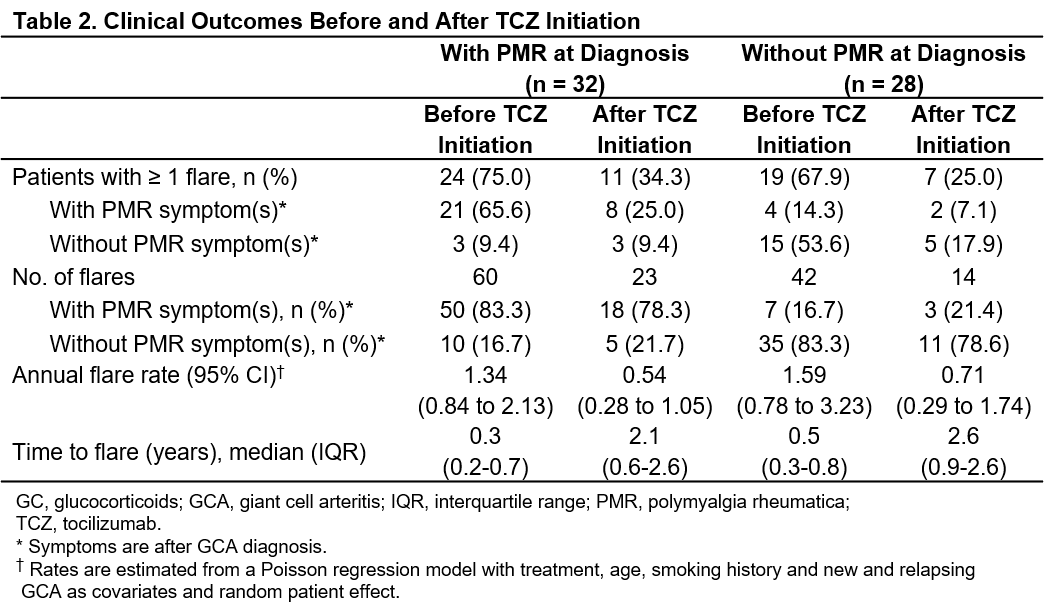
Results: A total of 60 patients with GCA (71.7% female; mean age, 69.3 years) were followed for a mean (SD) of 2.3 (2.2) years. At diagnosis, 32 had PMR symptoms (2 patients with PMR symptoms only). Table 1 shows baseline patient characteristics. Among patients with PMR at diagnosis, 75% (n = 24) had a total of 60 flares before TCZ initiation and 34.3% (n = 11) had a total of 23 flares after TCZ initiation (Table 2). Among 28 patients without PMR at diagnosis, 67.9% (n = 19) had a total of 42 flares before TCZ initiation and 25% (n = 7) had a total of 14 flares after TCZ initiation (Table 2). TCZ was associated with a significant reduction in the annual flare rate both in patients with PMR symptoms (P = 0.003) and without PMR symptoms (P = 0.03) at diagnosis (Table 2). Median time to flare was significantly longer after initiation of TCZ in patients with PMR symptoms (hazard ratio [HR] [95% CI]: 0.18 [0.06 to 0.53]; P = 0.002) and without PMR symptoms (HR [95% CI]: 0.21 [0.05 to 0.87]; P = 0.032) at diagnosis. Before TCZ initiation, PMR symptoms were observed in 55.9% of flares (57/102) occurring in 43 patients (71.1%). After TCZ initiation, PMR symptoms were observed in 56.8% of flares (21/37) occurring in 18 patients (30%).
Conclusion: TCZ improved clinical outcomes in patients with GCA regardless of the presence or absence of PMR symptoms at diagnosis. These real-world findings suggest that TCZ is also effective in patients with GCA who have symptoms of PMR.
Reference:
- Buttgereit F, et al. JAMA. 2016;315(22):2442-2458.
Acknowledgements: This study was funded by Genentech, Inc. Support for third-party writing assistance, furnished by Health Interactions, Inc, was provided by Genentech, Inc.
To cite this abstract in AMA style:
Unizony S, Spiera R, Pei J, Sidiropoulos P, Best J, Stone J. Clinical Outcomes of Patients with Giant Cell Arteritis and Polymyalgia Rheumatica Symptoms Treated with Tocilizumab in Routine Clinical Practice [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinical-outcomes-of-patients-with-giant-cell-arteritis-and-polymyalgia-rheumatica-symptoms-treated-with-tocilizumab-in-routine-clinical-practice/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-of-patients-with-giant-cell-arteritis-and-polymyalgia-rheumatica-symptoms-treated-with-tocilizumab-in-routine-clinical-practice/