Session Information
Date: Tuesday, November 12, 2019
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III: Giant Cell Arteritis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with giant cell arteritis (GCA) treated with tocilizumab (TCZ) plus prednisone tapering achieved higher rates of sustained glucocorticoid (GC)-free remission and had lower cumulative GC doses than patients treated with prednisone tapering alone in part 1 of the 52-week, double-blind GiACTA trial.1 During therapy, patients who received weekly TCZ reported improvement in the 36-item Short-Form Health Survey (SF-36) Mental Component Summary (MCS) and Physical Component Summary scores and FACIT-Fatigue scores that were statistically significant and clinically meaningful compared with patients who received prednisone alone.2 The objective of this analysis was to analyze whether such benefit in SF-36 MCS was maintained in patients originally assigned to receive TCZ in comparison with those originally assigned to receive placebo (PBO) plus a 26- or 52-week prednisone taper (PBO group) among patients who achieved clinical remission at week 52 and maintained treatment-free clinical remission in the 2-year, long-term extension of GiACTA.
Methods: At the end of part 1 of GiACTA, patients entered open-label part 2, in which GCA therapy (including initiation/termination of open-label TCZ and/or GCs) was given at the investigator’s discretion according to disease status. The change from baseline in SF-36 MCS score was compared for combined original TCZ (n = 33) and PBO (n = 17) patients who achieved clinical remission at week 52 and maintained treatment-free (no TCZ or GCs) clinical remission in part 2 using a repeated-measures model. The minimal clinically important difference (MCID) for SF-36 MCS is >2.5.3
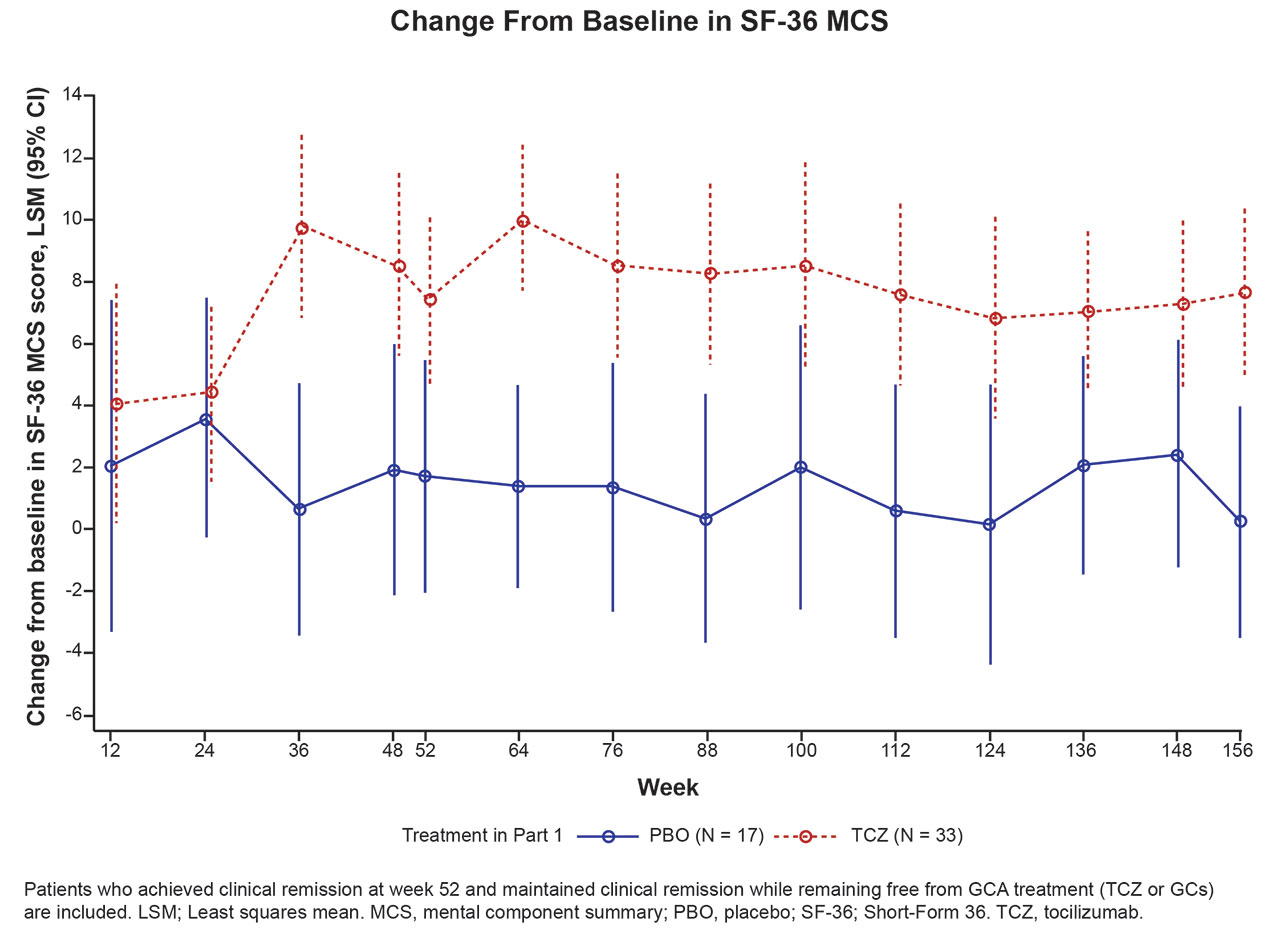
Results: During treatment, SF-36 MCS scores in all 50 patients who maintained treatment-free clinical remission in part 2 had diverged between the TCZ and PBO groups as early as 36 weeks after baseline, with greater improvements evident in the TCZ group (Figure 1). The difference in least squares mean (LSM) change between TCZ and PBO was statistically significant at week 52 (p = 0.016) and maintained at weeks 100 (p = 0.023) and 156 (p = 0.0019). The LSM difference (95% CI) between TCZ and PBO at weeks 52, 100, and 156 was 5.6 (1.1-10.2), 6.5 (0.9-12.1), and 7.4 (2.9-11.9), respectively, exceeding the MCID.
Conclusion: Among patients who maintained treatment-free clinical remission during part 2 of GiACTA, those originally assigned to receive TCZ plus a prednisone taper during part 1 maintained statistically significant and clinically meaningful improvements in SF-36 MCS up to week 156 compared with those originally assigned to receive PBO plus a prednisone taper in part 1. This was true even though neither of the patient groups received TCZ or GC treatment after they achieved clinical remission at week 52.
References: 1. Stone JH et al. N Engl J Med 2017;377:317-328. 2. Strand V et al. Arthritis Res Ther 2019;21:64. 3. Lubeck DP. Pharmacoeconomics 2004;22:27-38.
To cite this abstract in AMA style:
Stone J, Han J, Unizony S, Aringer M, Blockmans D, Brouwer E, Cid M, Dasgupta B, Rech J, Salvarani C, Spiera R, Bao M. Maintained Benefit in Health-Related Quality of Life of Patients with Giant Cell Arteritis Treated with Tocilizumab Plus Prednisone Tapering: Results from the Open-Label, Long-Term Extension of a Phase 3 Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/maintained-benefit-in-health-related-quality-of-life-of-patients-with-giant-cell-arteritis-treated-with-tocilizumab-plus-prednisone-tapering-results-from-the-open-label-long-term-extension-of-a-phas/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/maintained-benefit-in-health-related-quality-of-life-of-patients-with-giant-cell-arteritis-treated-with-tocilizumab-plus-prednisone-tapering-results-from-the-open-label-long-term-extension-of-a-phas/