Session Information
Date: Tuesday, November 12, 2019
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III: Giant Cell Arteritis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Giant cell arteritis (GCA) has traditionally been diagnosed by a combination of symptoms, clinical findings, laboratory results, and temporal artery biopsy. More recently, imaging studies have proven to be useful for the diagnosis. The purpose of our study was to describe the diagnostic workup for GCA in clinical practice among Medicare beneficiaries in the United States.
Methods: We conducted a cohort study of Medicare beneficiaries enrolled between 2011 and 2016, using a 5% representative sample. Beneficiaries with incident suspected GCA were identified by requiring at least one year of continuous enrollment in Part A and B prior to the first GCA-related claim, and the specialty of the provider who filed the claim was determined by the referring physician NPI number. We estimated the proportion of beneficiaries with procedures 90 days before or after the initial GCA claim, including temporal artery biopsy (TAB), temporal artery (TA) ultrasound (US), other imaging including MRA, CTA, and PET/CT of different vascular territories, and laboratory tests including CRP and ESR. Diagnosis of polymyalgia rheumatica (PMR) the year before or after the initial GCA claim was also ascertained. Agresti and Coull 95% confidence intervals were calculated for the proportions.
Results: Incident suspected GCA was identified in 9,159 beneficiaries; 71.6% women, 88.1% white, 7.7% African American, with mean age 77.0 (SD: 9.2). Initial GCA-related claims were filed by primary care providers (43.0%), ophthalmologists (20.4%), neurologists (12.6%), surgeons (6.1%), rheumatologists (4.9%), and other types of providers (13.0%); provider specialty from the initial claim was not available for 536 beneficiaries. Among beneficiaries with incident suspected GCA, 19.8% (95%CI: 19.0, 20.6) underwent TAB, 1.2% (95%CI: 1.0, 1.4) had US of the TA, and 10.0% (95%C: 9.4, 10.6) underwent other imaging within 90 days before or after the initial claim. Laboratory tests included CRP (19.4%; 95%CI: 18.6, 20.3) and ESR (27.6%; 95%CI: 26.6, 28.5). PMR was suspected or diagnosed in the year before or after the initial GCA claim for 11.6% (95%CI: 11.0, 12.3), though notably higher for patients seen by rheumatologists (32.8%; 95%CI: 28.5, 37.4). Among beneficiaries who underwent TAB, 46.3% (95CI: 43.6, 49.0) had a GCA diagnosis claim during the 30 days following the TAB, potentially reflecting the biopsy result; among patients seen by rheumatologists, 57.1% (95%CI: 46.5, 67.2) had a GCA diagnosis claim within 30 days after the TAB. Details by provider specialty are shown in Table 1.
Conclusion: Medicare beneficiaries with suspected GCA did not commonly undergo TAB, imaging studies, and laboratory tests for ESR and CRP between 2012 and 2016, suggesting that clinical criteria were primarily used during diagnostic workup. GCA claims were most often first filed by primary care physicians and uncommonly first filed by rheumatologists. Our results suggest that guidelines for diagnosis of GCA in the United States are warranted. Development of Fast-Track Clinics for GCA to assure rapid access to a multidisciplinary team lead by rheumatologists are also highly recommended.
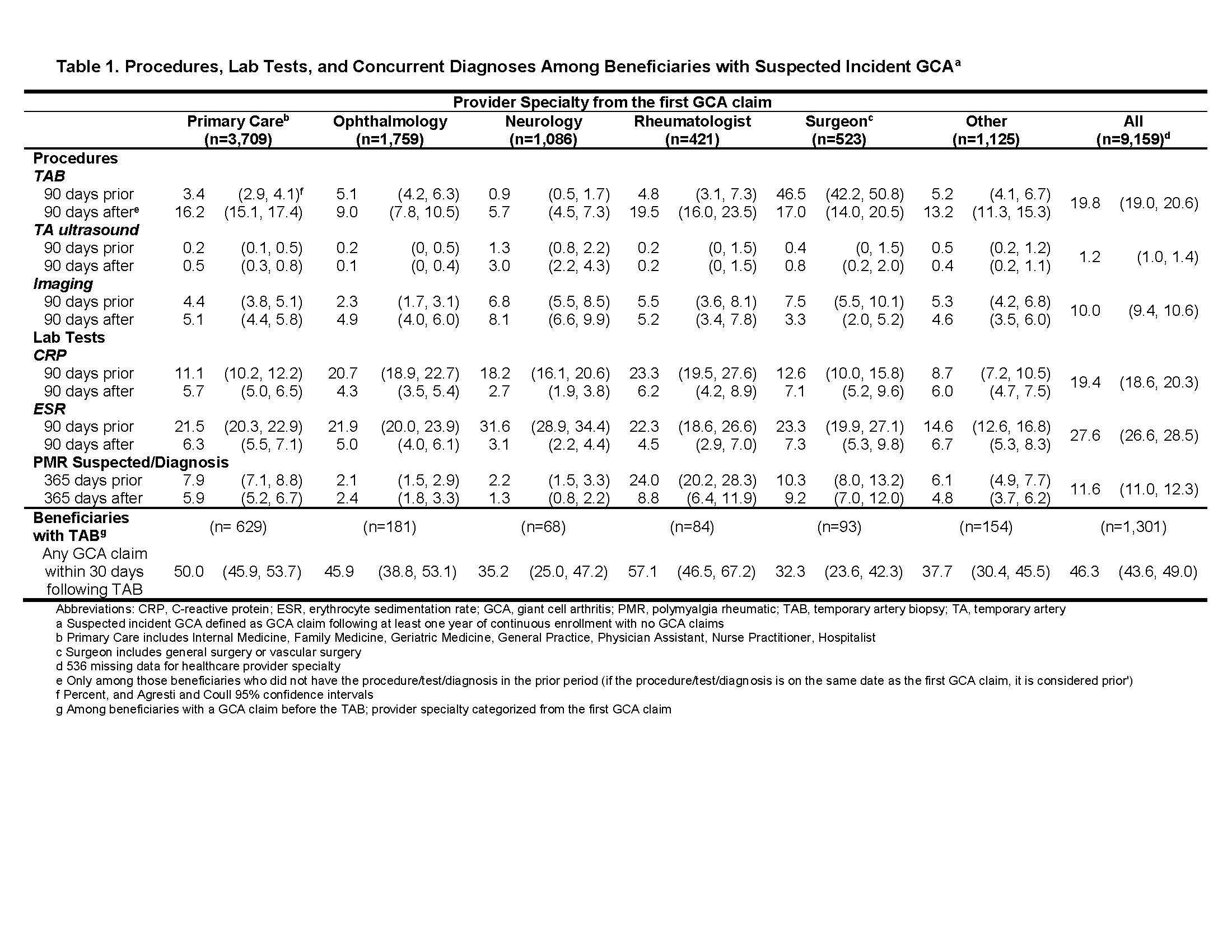
Table 1_Medicare_GCA_ACR Abstract 2019_Submitted_PDF
To cite this abstract in AMA style:
Rodriguez-Pla A, Zhou L, Ashbeck E, Kwoh C. Giant Cell Arteritis Diagnostic Workup Among Medicare Beneficiaries [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/giant-cell-arteritis-diagnostic-workup-among-medicare-beneficiaries/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/giant-cell-arteritis-diagnostic-workup-among-medicare-beneficiaries/
