Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The impact of glucocorticoid (GC) use on major organ damage in SLE patients has not been formally studied by amalgamating the relevant data published in the literature over the past 40 years. We aimed to study the association between GC use and the occurrence of major organ damage in SLE patients by performing meta-analyses of observational studies published between 1970 and December 2018.
Methods: Literature search on PubMed (from 1966 to December 2018) for prevalence and longitudinal studies which reported GC exposure (proportion of GC users in the cohort [%GC use] and/or GC use in defined doses) and the occurrence (prevalence/incidence) of major organ damage in SLE patients using the keywords “cataract”, “cerebrovascular” (CVA), “stroke”, “cardiovascular” (CVS), “angina”, “myocardial infarction” (MI), “coronary artery bypass”, “osteoporosis”, “avascular necrosis” (AVN) and “osteonecrosis” in respective combinations with “lupus” was conducted. Studies with sample size < 50 and observation duration < 12 months were excluded. The logit of the proportion of patients with disease damage was modelled as a random effect in the meta-analysis, which was employed to study the association between the proportion of patients with organ damage and variables of GC use (mean daily [mg/day] and cumulative [gm] prednisone [PDN] doses and %GC use). A 2-stage estimation of the random-effects logistic regression models was used with restricted maximum likelihood estimation. Univariate associations between organ damage and moderators were examined for statistical significance, and variables related to GC use were adjusted for SLE disease duration in multivariate models if their univariate P values were < 0.2.
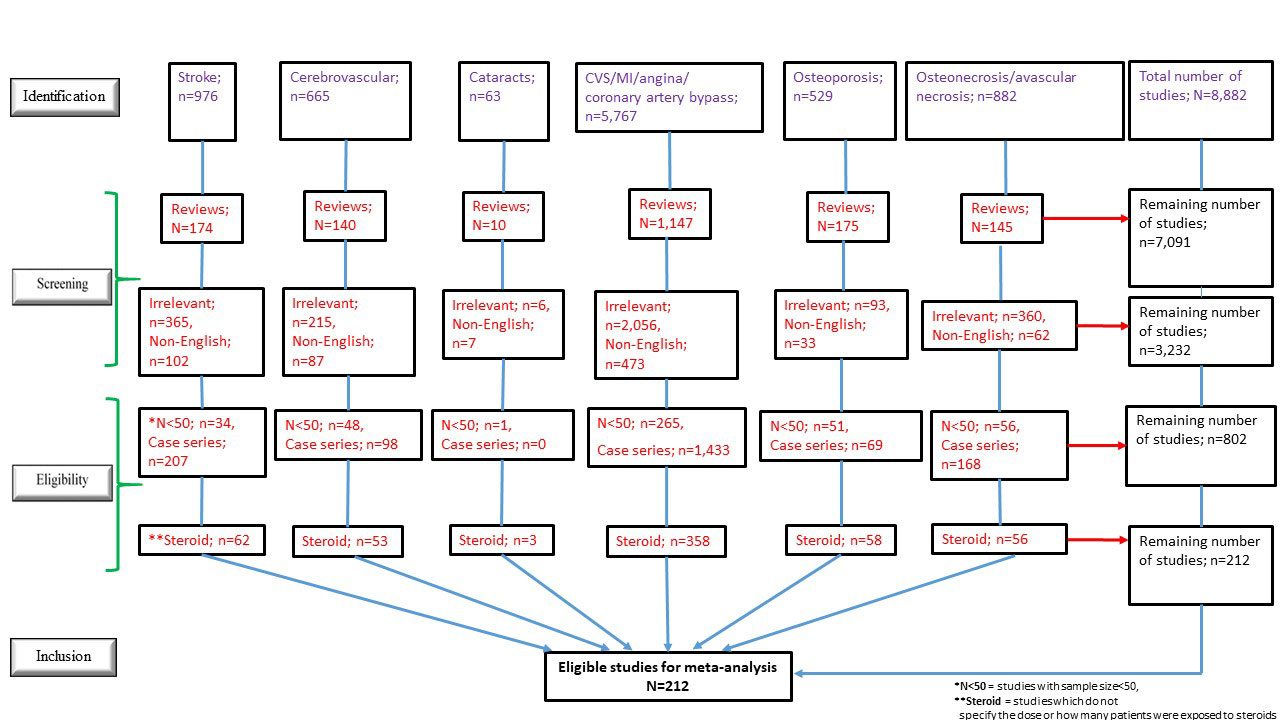
Results: Out of 8,882 publications screened, 212 articles involving 205,619 SLE patients were eligible for the meta-analyses (Figure 1), of which 97 were prevalence and 115 were longitudinal studies. Univariate analyses of prevalence studies revealed that mean daily PDN dose (odds ratio [OR]=1.10, p=0.007) and lower proportion of female in the cohort (OR=0.002, p=0.002) were associated with the prevalence of overall CVS events. Mean daily PDN dose (OR=1.52, p< 0.001) and %GC use (OR=2,255.2, p< 0.001) were associated with the prevalence of AVN. A significant association between cumulative PDN dose and prevalence of CVA was found after multivariate adjustment for SLE disease duration (OR=1.07, p=0.017). In longitudinal studies, a significant association was identified between cumulative PDN dose and incidence of cataracts after adjustment for SLE disease duration (OR=1.04, p=0.013). While the incidence of MI in SLE patients has dropped over the past 40 years (OR=0.94, p=0.002), it was associated with % GC use after adjustment for SLE disease duration (OR=8.18, p=0.012). Interestingly, significant univariate associations were found between antimalarial use and lower prevalence of MI (OR=0.05, p=0.002) and lower incidence of CVA (OR=0.20, p=0.032).
Conclusion: Independent of SLE disease duration, cumulative PDN dose was associated with higher prevalence of CVA and incidence of cataracts, and higher incidence of MI was associated with overall GC use.
To cite this abstract in AMA style:
Mak A, Cheung M, Leong W, Dharmadhikari B, Kow N, Petri M, Manzi S, Clarke A, Aranow C, Arnaud L, Askanase A, Bae S, Bernatsky S, Bruce I, Buyon J, Chatham W, Costedoat-Chalumeau N, Dooley M, Fortin P, Ginzler E, Gladman D, Gordon C, Hanly J, Inanc M, Isenberg D, Jacobsen S, James J, Jönsen A, Kalunian K, Kamen D, Lim S, Morand E, Peschken C, Pons-Estel B, Rahman A, Ramsey-Goldman R, Romero-Diaz J, Ruiz-Irastorza G, Sanchez-Guerrero J, Steinsson K, Svenungsson E, Urowitz M, van Vollenhoven R, Vinet E, Voskuyl A, Wallace D, Alarcón G. Glucocorticosteroid Usage and Major Organ Damage in Patients with Systemic Lupus Erythematosus – Meta-analyses of Observational Studies Published Between 1979 and 2018 [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/glucocorticosteroid-usage-and-major-organ-damage-in-patients-with-systemic-lupus-erythematosus-meta-analyses-of-observational-studies-published-between-1979-and-2018/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticosteroid-usage-and-major-organ-damage-in-patients-with-systemic-lupus-erythematosus-meta-analyses-of-observational-studies-published-between-1979-and-2018/