Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
Hydroxychloroquine (HCQ) use in SLE has been associated with a lower risk of end-organ damage, SLE flares, and thrombosis1,2. However the benefits of HCQ among SLE with end stage renal disease (ESRD) are less clear3. Despite HCQ benefits, fewer than 30% of SLE continue HCQ after ESRD onset3. On the other hand, there is an increased risk of HCQ toxicity among SLE-ESRD4. It has not been studied what factors are associated with HCQ use after ESRD. Understanding these factors may inform future studies assessing the safety and efficacy of HCQ in SLE-ESRD and address the “confounding by-indication” bias (i.e. different treatments are intentionally chosen for patients with different prognoses) when analyzing retrospective data regarding HCQ use in SLE patients with ESRD. Therefore, the objective of this study was to determine the factors associated with HCQ use among SLE patients with ESRD.
Methods:
We performed a retrospective chart review of SLE patients with ESRD at a single tertiary care center between 2010-2017. All included patients met ACR and/or SLICC criteria for SLE and had at least one visit with rheumatology, nephrology, dermatology or primary care, before and after the development of ESRD. SLE-related symptoms, serologic markers of disease activity, and rheumatology visits were identified, both pre- and post-ESRD onset. Transplanted patients were excluded at the time of their first renal transplant.
Results: A total of 69 patients were included, 58 had pre-ESRD data. Of these patients, 33/58 (57%) were taking HCQ prior to ESRD onset. Following the diagnosis of ESRD, 40/69 (58%) were prescribed HCQ within six months after ESRD onset. Of these, six discontinued HCQ by the last documented visit, and one patient initiated HCQ six months after ESRD onset (prescribed by a rheumatologist). At the last documented visit, 35/69 patients (51%) had an active HCQ prescription. Patients taking HCQ were younger, more likely to be followed by a rheumatologist, had a higher frequency of documented arthritis, higher frequency of corticosteroid use and immunosuppressive medication use (Table 1). A history of oral ulcers, cytopenias, and elevated levels of dsDNA at any point (either pre or post-ESRD onset) was not significantly associated with HCQ use at the last visit.
Conclusion: HCQ is more likely to be continued among patients with signs of persistently active SLE. HCQ was more likely to be prescribed by a rheumatologist and was associated with the presence of arthritis. None of the serology markers of disease activity were associated with HCQ prescription. Limited systemic evaluation and/or documentation by the different providers may have resulted in under-recognition and under-reporting of some of the SLE symptoms. However, these findings reflect the “real-world” experience with HCQ use after ESRD in a large tertiary care center.
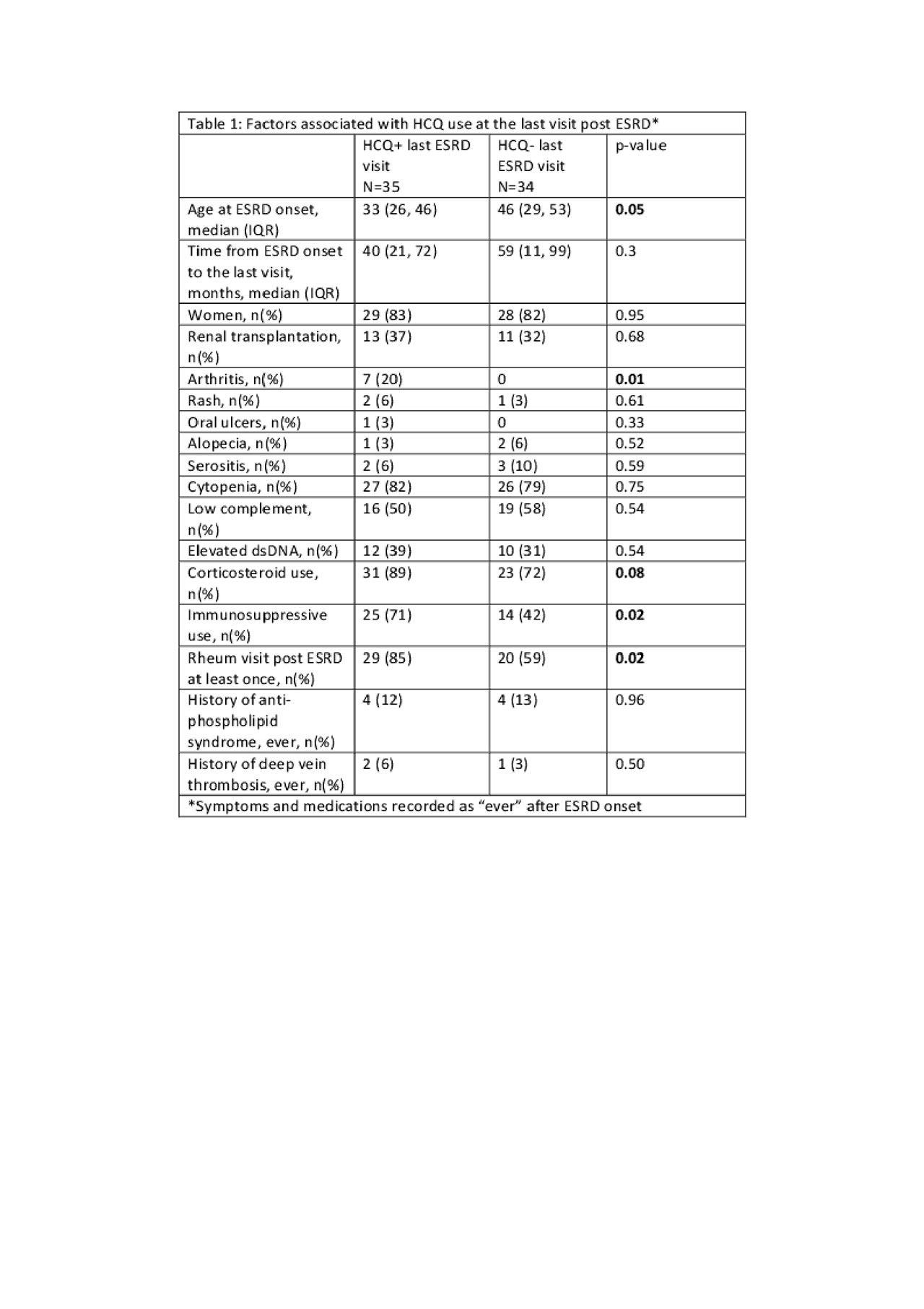
Table 1. Factors associated with HCQ use at the last visit post ESRD
To cite this abstract in AMA style:
Salgado Guerrero M, Londono Jimenez A, Dobrowolsky C, Wang S, Mowrey W, Broder A. Factors Associated with Hydroxychloroquine Use in Systemic Lupus Erythematosus Patients with End Stage Renal Disease [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/factors-associated-with-hydroxychloroquine-use-in-systemic-lupus-erythematosus-patients-with-end-stage-renal-disease/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-hydroxychloroquine-use-in-systemic-lupus-erythematosus-patients-with-end-stage-renal-disease/
