Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Chloroquine (CQ) and Hydroxychloroquine (HCQ) have been used for years during pregnancy for multiple indications (malaria prevention, autoimmune disorders, etc.…). Recently, some countries face restrictions to prescribe these drugs during pregnancy, due to report of ocular toxicity in animal models and potential genotoxicity. Change of the summary of product characteristics could significantly impact the adherence to this essential treatment for pregnancy in CQ/HCQ treated patients. The aim of this study was to perform a systematic review and a meta-analysis of fetal malformation rate under CQ and HCQ (all indications) during pregnancy. In the subgroup of patients suffering from an autoimmune disease, we performed an analysis of CQ and HCQ efficacy on maternal and pregnancy outcomes.
Methods:
We systematically searched literature from inception to February 2 2019 (via Pubmed, Embase and abstracts from ACR and EULAR congresses) for studies that compare fetal malformation rate and pregnancy outcomes of CQ/HCQ versus placebo. Two independent reviewers carried out the review. A meta-analysis was performed, using the inverse variance approach, to estimate risk differences for malformation rate with its 95% confidence interval. Maternal and pregnancy outcomes will be analyzed with global odds-ratios and their 95% confidence interval. Heterogeneity was tested with Cochran’s Q-test and I2 value, and a random effect model was performed if needed. Revman software was used, considering a p-value threshold of 5%.
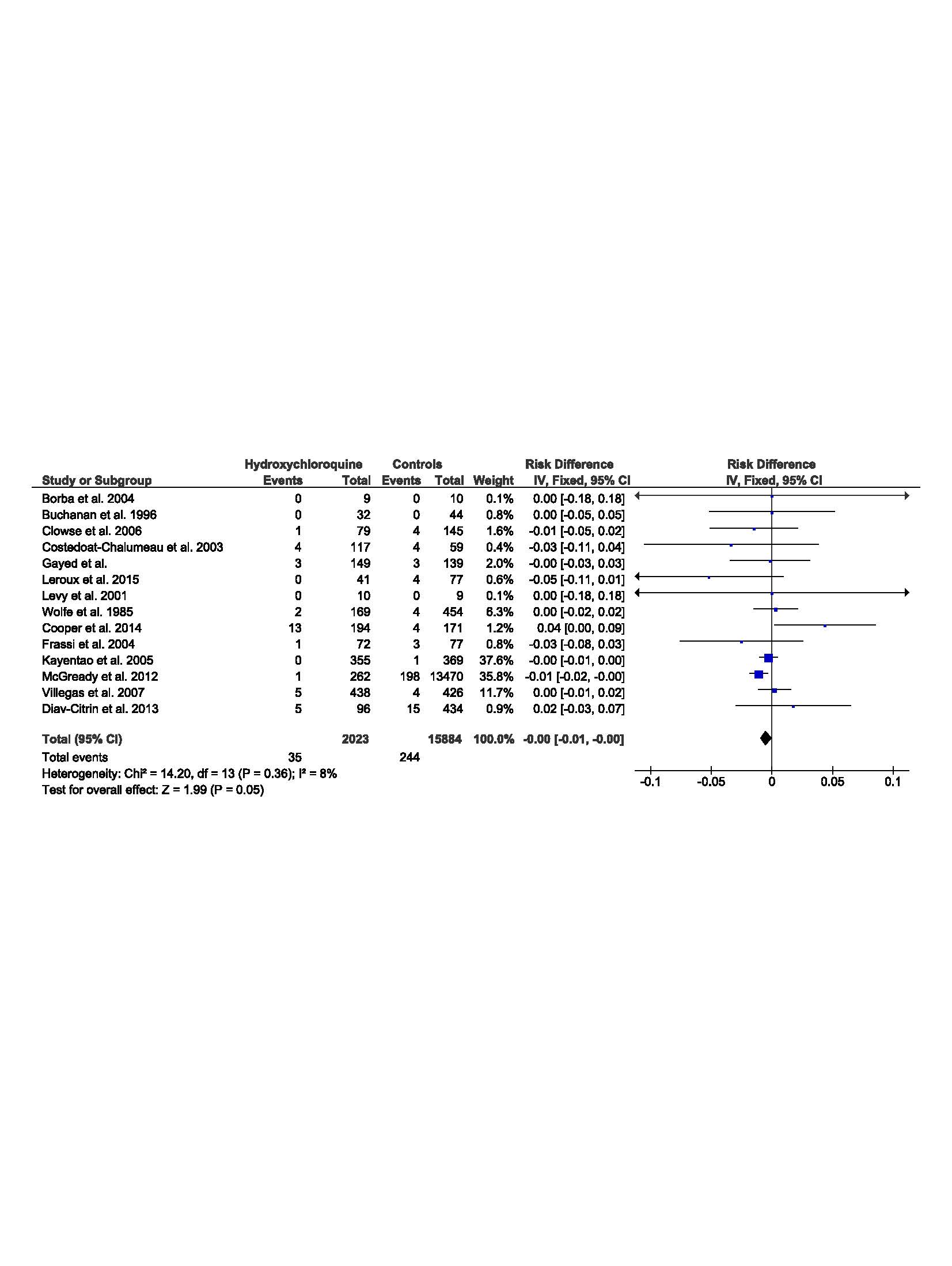
Results: From 2801 articles, the literature search revealed 90 articles and abstracts of potential interest, and further examination resulted in 14 studies fulfilling required criteria, with 12 cohort studies (7 prospective and 5 retrospective), and 2 randomized controlled trials. The selected articles include a total of 2023 exposed children of mothers treated by CQ/HCQ for an autoimmune disease (lupus, Sharp syndrome, Sjogren…) or malaria indication (treatment or prevention), compared to 15884 children in the control group.
The meta-analysis did not highlight any increase of malformation rate between the two groups (DR=0,00 [-0.01, 0.00]95%; p=0.05, I2=8%).
In the secondary analysis on the maternofetal outcomes within studies on autoimmune diseases, the analyses did not reveal any difference between the two groups for occurrence of disease flare (OR=0.89 [0.21, 3.77]95%; p=0.88; I2=79%), pre-eclampsia (OR=1.14 [0.59, 2.23]95%; p=0.69; I2=5%), prematurity (OR=1.17 [0.46, 2.97]95%; p=0.75; I2=81%), foetal growth restriction (OR=0.54 [0.10, 2.82]95%; p=0.46; I2=89%), or congenital auriculo-ventricular block (OR=0.61 [0.17, 2.1]95%; p=0.43; I2=0%). Conversely, the systematic literature reviews identified several cohort studies confirming the importance of HCQ in preventing lupus flares.
Conclusion:
In our study, prenatal exposure to CQ or HCQ was not associated with any congenital abnormalities. HCQ should be continued in pregnancy for maintenance of remission or treatment of a disease flare.
To cite this abstract in AMA style:
Naveau T, Lichau O, Barnetche T, Schaeverbeke T, Lazaro E, Truchetet M, Richez C. Safety of Chloroquine and Hydroxychloroquine During Pregnancy: A Systematic Review and Meta-Analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/safety-of-chloroquine-and-hydroxychloroquine-during-pregnancy-a-systematic-review-and-meta-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-chloroquine-and-hydroxychloroquine-during-pregnancy-a-systematic-review-and-meta-analysis/