Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept (CTLA-4-Ig) targets the CD80/CD86:CD28 co-stimulatory pathway required for full T-cell activation and T-cell dependent activation of B-cells. The Abatacept Sjögren Active Patients phase III (ASAPIII) trial assessed the efficacy and safety of treatment with subcutaneous abatacept in patients with early active primary Sjögren’s syndrome (pSS). In the double blind phase, no difference was found in the primary endpoint, EULAR Sjögren’s syndrome disease activity score (ESSDAI) after 24 weeks, but a higher number of patients receiving abatacept reached the minimal clinically important change in EULAR Sjögren Syndrome Patient Reported Index (ESSPRI). B-cell hyperactivity was decreased by abatacept. The aim of the current analysis is to evaluate efficacy and safety of extended treatment with abatacept after 48 weeks.
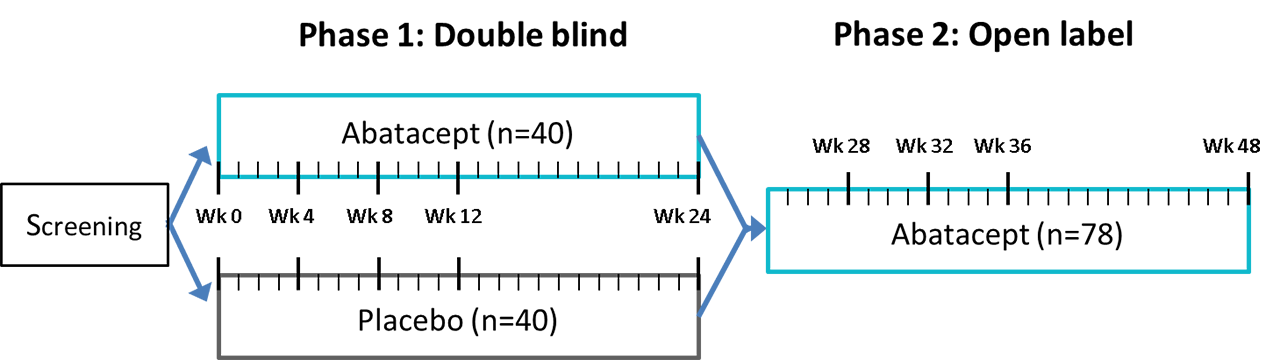
Methods: The ASAP-III study is a monocenter, investigator-initiated, double-blind, placebo-controlled trial with an open label extension phase (NCT02067910). ASAP-III included 80 adult patients with biopsy-proven pSS, fulfilling the AECG and ACR/EULAR criteria, with a disease duration of ≤7 years and moderate to high (ESSDAI ≥5). After receiving weekly subcutaneous injections with abatacept (125 mg) or placebo for 24 weeks, all patients were treated with abatacept for another 24 weeks (figure 1). Concomitant use of other DMARDs including hydroxychloroquine was not permitted, with the exception of a stable dose of prednisone (≤10mg), and rescue therapy with prednisone or cyclophosphamide. At each visit (see figure 1), participants were evaluated by a multidisciplinary team of rheumatologists, ophthalmologists, and oral and maxillofacial surgeons. The primary endpoint was ESSDAI at 24 weeks. Secondary outcomes at 24 and 48 weeks included clinical, patient reported, functional, histological, laboratory, ultrasound, and microbiome parameters, and the occurrence of (serious) adverse events and treatment discontinuation.
Results: Patient characteristics at baseline and in week 24 are shown in table 1. Of 80 included patients, 78 were included in the extension phase. Two patients from the placebo arm were lost to follow up before week 24. Database lock for the extension phase is planned in September 2019. Subsequently, the first analyses of the week 24-48 results will be performed, focusing on ESSDAI, ESSPRI, quality of life (EQ-5D-5L), unstimulated and stimulated whole salivary flow (UWS, SWS), Schirmer’s test, Ocular Staining Score (OSS), serological parameters (rheumatoid factor, IgG), and safety parameters.
Conclusion: The ASAP-III trial was designed to assess the clinical efficacy and safety of subcutaneous abatacept in pSS patients with short disease duration and active disease. Results of week 24-48, the open-label extension phase, are available at the ACR 2019 annual meeting.
To cite this abstract in AMA style:
van Nimwegen J, Mossel E, Wijnsma R, van Zuiden G, Delli K, Stel A, van der vegt B, Haacke E, Olie L, Los L, Verstappen G, Pringle S, Spijkervet F, Kroese F, Vissink A, Arends S, Bootsma H. Efficacy and Safety of Abatacept in Patients with Early Active Primary Sjögren’s Syndrome – Open-label Extension Phase of a Randomized Controlled Phase III Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/efficacy-and-safety-of-abatacept-in-patients-with-early-active-primary-sjogrens-syndrome-open-label-extension-phase-of-a-randomized-controlled-phase-iii-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-and-safety-of-abatacept-in-patients-with-early-active-primary-sjogrens-syndrome-open-label-extension-phase-of-a-randomized-controlled-phase-iii-trial/