Session Information
Date: Tuesday, November 12, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Patient Preferences, Beliefs, & Experiences
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The treat to target (T2T) treatment strategy is associated with better clinical outcomes in patients with rheumatoid arthritis (RA). We have previously found that a direct-to-patient video intervention aimed to increase knowledge about T2T was associated with increased willingness to adopt RA treatment. The goal of this study was to evaluate patient and clinical characteristics associated with improved willingness to adopt treatment.
Methods: The CONTROL-RA trial, as previously reported, enrolled participants with self-reported RA, with RAPID 3 >4 (range 0-10) and without potentially confounding conditions or who had been using disease modifying anti-rheumatic drugs (DMARDs) in the past 6 months. Outcomes were collected using compensated surveys. Participants completed a baseline survey, viewed the educational intervention and completed the follow up survey immediately after. Intervention group participants viewed up to 6 videos (minimum of 2, mean duration 2 min) on topics relevant to T2T. We performed a multivariate analysis, where the primary outcome was pre-post difference in patient-reported willingness to adopt treatment. The following factors were considered: extent of engagement (e.g. high engagement group watched >80% of each of the 6 videos vs low engagement group watched less), age, sex, race, prior biologic/DMARD use, general health, health literacy, familiarity with T2T, patient global assessment, patient acceptable symptom state (PASS), decision conflict about treatment change (decision conflict scale, lower score is better), readiness for treatment change (precaution adoption process model) and personal attitudes favoring medications (10 items surveying patient attitudes regarding acceptable medication risks vs benefits; higher score is more favorable).
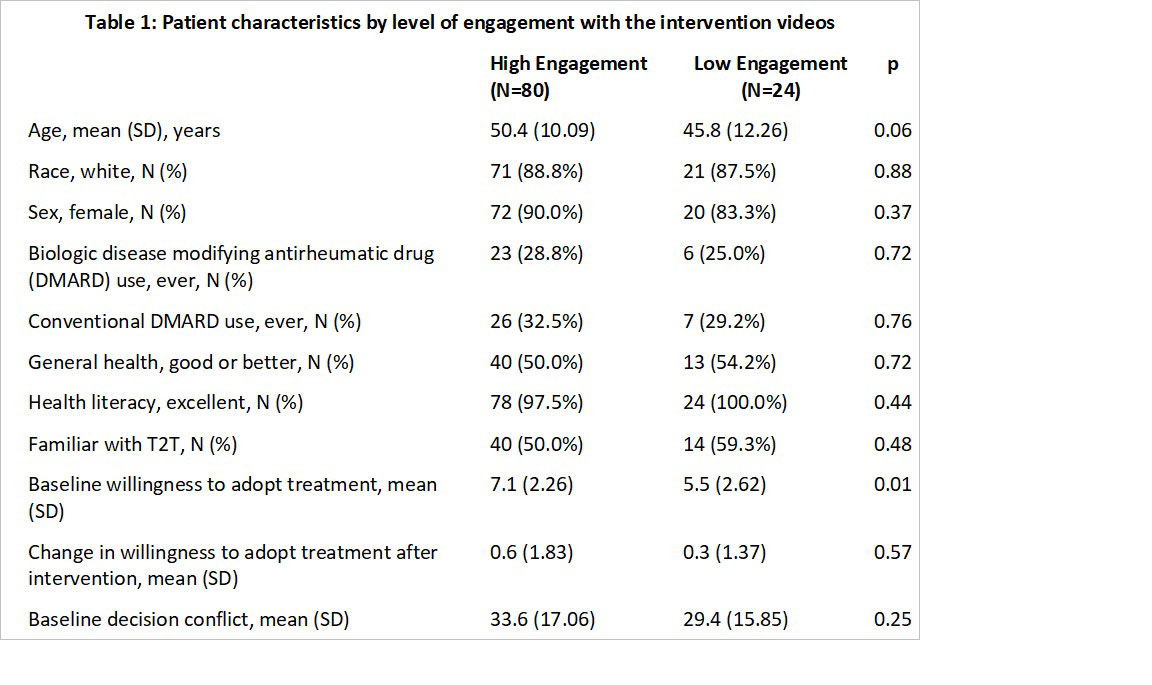
Results: Responses of 104 participants in the intervention group were analyzed (Table 1). Participants were 92% white, 92% women, with a mean age of 49 years. A total of 80 (77%) participants were classified in the high engagement group. Participants classified in the high engagement group had higher baseline willingness to adopt treatment than those in the low engagement group (7.1 vs 5.5, p=0.006). The pre-post difference in willingness to adopt treatment was 0.6 in the high engagement group and 0.3 in the low engagement group (p = 0.57). In multivariable models the level of engagement with the intervention was not associated with improved willingness to adopt treatment (p=0.14). Lower baseline level of willingness to change RA treatment, older age and personal attitudes favoring medications were associated with improved willingness to adopt RA treatment (Table 2).
Conclusion: We observed a high level of engagement and were thus not able to discern a dose-response relationship between our intervention and improved willingness after the intervention. Interestingly, those with higher willingness to change treatment were more likely to view the educational intervention, suggesting that those who are less open to treatment change may need additional support to get involved with this type of educational program.
To cite this abstract in AMA style:
Taylor A, Chen L, O'Beirne R, Melnick J, Ruderman E, Curtis J, Danila M. Patient and Clinical Characteristics Associated with Increased Willingness to Adopt RA Treatment After an Educational Intervention: An Analysis of the Confident Treatment Decisions for Living with Rheumatoid Arthritis (CONTROL-RA) Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/patient-and-clinical-characteristics-associated-with-increased-willingness-to-adopt-ra-treatment-after-an-educational-intervention-an-analysis-of-the-confident-treatment-decisions-for-living-with-rhe/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-and-clinical-characteristics-associated-with-increased-willingness-to-adopt-ra-treatment-after-an-educational-intervention-an-analysis-of-the-confident-treatment-decisions-for-living-with-rhe/